Nicotine, C 10 H 14 N 2 , has two basic nitrogen atoms (Figure 16.12), and both
Question:
Nicotine, C10H14N2, has two basic nitrogen atoms (Figure 16.12), and both can react with water.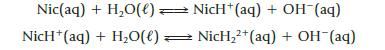
Kb1 is 7.0 × 10−7 and Kb2 is 1.1 × 10−10. Calculate the approximate pH of a 0.020 M solution.
Data given in Table 16.2


Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





