Oxalic acid is a relatively weak diprotic acid. Calculate the equilibrium constant for the reaction shown below
Question:
Oxalic acid is a relatively weak diprotic acid. Calculate the equilibrium constant for the reaction shown below from Kal and Ka2. (See Appendix H for the required Ka values.)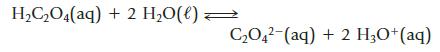
Data given in Appendix H
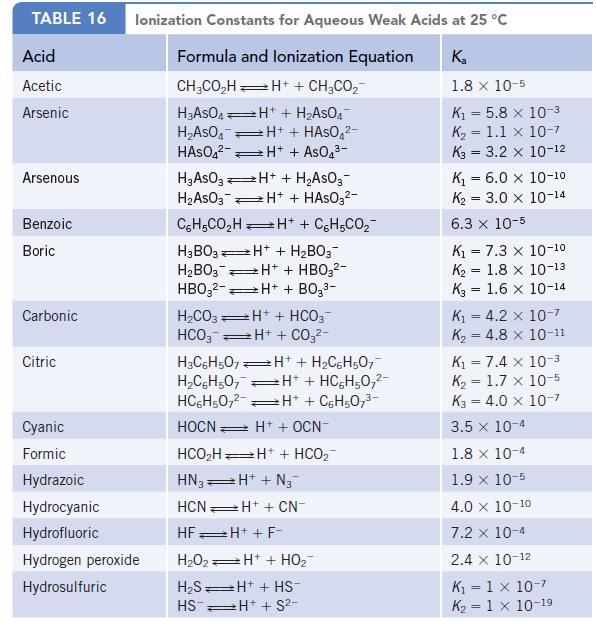
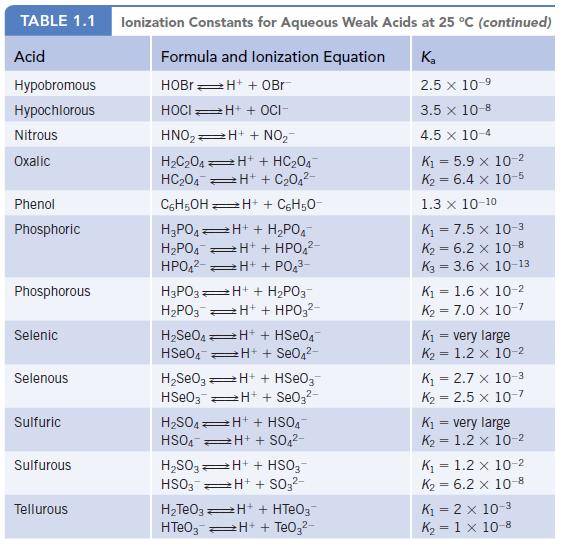
Transcribed Image Text:
H,C,O4(aq) + 2 H,O(l) < C,O4?-(aq) + 2 H,O+(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The odor of fish is due primarily to amines, especially methylamine (CH3NH2). Fish is often served with a wedge of lemon, which contains citric acid. The amine and the acid react forming a product...
-
The following equilibrium constants have been determined for oxalic acid at 25°C: Calculate the equilibrium constant for the following reaction at the same temperature: H2C204(aq)H(aq) HC20 (aq)...
-
The phase diagram for SO2 is shown here. (a) What does this diagram tell you about the enthalpy change in the reaction SO2(I) SO2(g)? (b) Calculate the equilibrium constant for this reaction at 100...
-
Danis Inc is an American firm. The company will receive 626,000 British pounds (GBP) from one of its trading partners in 30 days. The company has obtained an analyst report for possible foreign...
-
What are some of the possible reasons Scott did not seek or receive advice from her immediate supervisor? Sue Ann Scott was a receptionist at the headquarters of a large corporation. A high school...
-
What is a performance appraisal? What are the most common mistakes managers make in performance appraisals? What should small business managers do to avoid making those mistakes?
-
Consider the following exponential probability density function. a. Write the formula for P(x x0). b. Find P(x 2). c. Find P(x 3). d. Find P(x 5). e. Find P(2 x 5). Applications
-
The Project Data file provided for you contains information that you may use to help you complete certain questions. You will be directed to this file as required in the questions below. Canaans...
-
Please answer this question and show steps. Check my work 3 Millco Inc, acquired a machine that cost $1.200,000 early in 2019. The machine is expected to last for eight years, and its estimated...
-
Given the following solutions: (a) 0.1 M NH 3 (b) 0.1 M Na 2 CO 3 (c) 0.1 M NaCl (d) 0.1 M CH 3 CO 2 H (e) 0.1 M NH 4 Cl (f) 0.1 M NaCH 3 CO 2 (g) 0.1 M NH 4 CH 3 CO 2 (i) Which of the solutions are...
-
The base ethylamine (CH 3 CH 2 NH 2 ) has a K b of 4.3 10 4 . A closely related base, ethanolamine (HOCH 2 CH 2 NH 2 ), has a K b of 3.2 10 5 . (a) Which of the two bases is stronger? (b) Calculate...
-
Match each definition on the left with a mathematical expression or term on the right. Difference between the averages in two populations (a) t = -4.6 (b) t = 1.3 (c) µ 1 µ 2 (d) (e) n -...
-
2. Boxes A and B are being pulled to the right by a rope attached to box B. Box A sits on top of box B, and both boxes accelerate together to the right at a rate of 1.75 m/s. The masses and...
-
You bought a 15-kilogram sack of unshelled peanuts for your restaurant. You weigh the sack three times on a balance, with the following results: Trial Mass (kg) 1 15.02 2 15.49 3 15.91 The results...
-
Two hikers leave the same tent at a campground and go separate ways. One hiker walks 8 miles directly south to Ashville, and the other hiker walks 14 miles directly northwest (i.e., N45W) to...
-
City Feb. Cases March Cases April Cases New York 19 56 189 Los Angeles 6 12 201 Chicago 0 3 14 Houston 19 19 272 Philadelphia 0 1 5 Phoenix 23 78 289 San Antonio 6 9 95 San Diego 3 38 258 Dallas 4 13...
-
Maximize z = 2x+2y x+6y <30 4x + 2y 32 Subject to I 0 W O 0 Maximum is I = y= at
-
A squirrel weighing 1.2 pounds climbed a cylindrical three by following the helical path x = cos t, y = sin t, z = 4t, 0 ( t ( 8( (distance measured in feet). How much did it do? Use a line integral,...
-
(a) What do data breach notification laws require? (b) Why has this caused companies to think more about security?
-
Consider rotation about the C~C bond in ethane. A crude model for torsion about this bond is the free rotor model where rotation is considered unhindered. In this model the energy levels along the...
-
Inspection of the thermodynamic tables in the back of the text reveals that many molecules have quite similar constant volume heat capacities. a. The value of C V, m for Ar (g) at standard...
-
The molar constant volume heat capacity for I 2 (g) is 28.6 J mol 1 K 1 . What is the vibrational contribution to the heat capacity? You can assume that the contribution from the electronic degrees...
-
17. Winedark Sea Ltd sells prints of classic paintings. The prints are done on expensive paper and are quite costly. Pricing the prints to sell is hard because the popularity of a print is difficult...
-
plz be clear and specific with answer Sweeten Company had no jobs in progress at the beginning of March and no beginning inventories. The company has two manufacturing departments --Molding and...
-
The following T account balances are taken from Forester Corp. Manufacturing Overhead Work in Process 370,000 366,000 90,000 Finished Goods Cost of Goods Sold 75,000 450,000 Required: Prepare the...

Study smarter with the SolutionInn App


