The data below compare the strength of acetic acid with a related series of acids, where the
Question:
The data below compare the strength of acetic acid with a related series of acids, where the H atoms of the CH3 group in acetic acid are successively replaced by Br.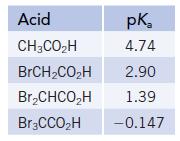
(a) What trend in acid strength do you observe as H is successively replaced by Br? Can you suggest a reason for this trend?
(b) Suppose each of the acids above was present as a 0.10 M aqueous solution. Which would have the highest pH? The lowest pH?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





