The formation of NO from N 2 and O 2 is unfavorable at 298 K, but it
Question:
The formation of NO from N2 and O2 is unfavorable at 298 K, but it becomes increasingly favored at high temperatures such as those in an automobile cylinder. Using data (ΔfH°, S° and ΔfG°) in Appendix L, calculate the equilibrium constant for the reaction ½ N2(g) + ½ O2(g) → NO(g) at 298 K and at 1000 K.
Data given in Appendix L
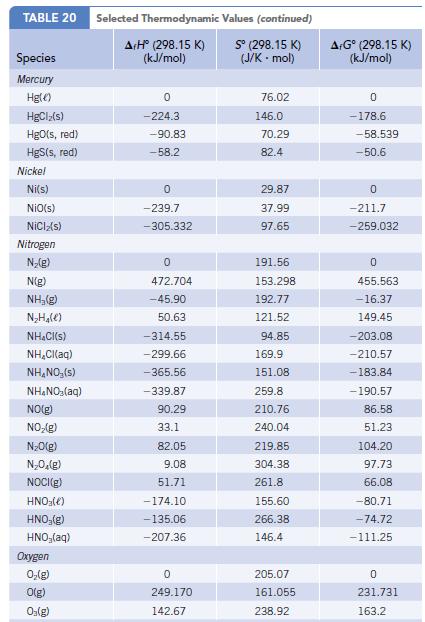
Transcribed Image Text:
TABLE 20 Species Mercury Hg() HgClz(s) HgO(s, red) HgS(s, red) Nickel Ni(s) NiO(s) NICIz(s) Nitrogen N₂(g) N(g) NH,(g) N₂H₂(e) NH₂Cl(s) NH₂Cl(aq) NH₂NO, (s) NH4NO₂(aq) NO(g) NO₂(g) N₂O(g) N₂O₂(g) NOCI(g) HNO3(e) HNO₂(g) HNO₂(aq) Oxygen 0₂(g) O(g) O₂(g) Selected Thermodynamic A+Hº (298.15 K) (kJ/mol) 0 -224.3 -90.83 -58.2 0 -239.7 -305.332 0 472.704 -45.90 50.63 -314.55 -299.66 -365.56 -339.87 90.29 33.1 82.05 9.08 51.71 -174.10 -135.06 -207.36 0 249.170 142.67 Values (continued) Sº (298.15 K) (J/K-mol) 76.02 146.0 70.29 82.4 29.87 37.99 97.65 191.56 153.298 192.77 121.52 94.85 169.9 151.08 259.8 210.76 240.04 219.85 304.38 261.8 155.60 266.38 146.4 205.07 161.055 238.92 A+Gº (298.15 K) (kJ/mol) 0 -178.6. -58.539 -50.6 0 -211.7 -259.032 0 455.563 -16.37 149.45 -203.08 -210.57 -183.84 -190.57 86.58 51.23 104.20 97.73 66.08 -80.71 -74.72 -111.25 0 231.731 163.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Formation of NO from N2 and O2 The formation of NO from N2 and O2 is an endothermic reaction meaning ...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Explain, using Le Chtelier's principle, why the equilibrium constant for the formation of NO from N2 and O2 increases with increasing temperature, whereas the equilibrium constant for the formation...
-
The equilibrium constant (KP) for the formation of the air pollutant nitric oxide (NO) in an automobile engine at 530°C is 2.9 Ã 10-11: (a) Calculate the partial pressure of NO under these...
-
Write sentences for the long-term direction and strategic path that management intends to follow. "Where we are headed?" and should explain why the direction in which you intend to point the company...
-
Use the following information for this question: Taxable income Marginal tax rate 15% 25% 34% 39% 34% 35% S S 0-S 50,000 75,000 50,000-$ S 75,000 $100,000 $ 100,000-S 335,000 S 335,000-$10,000,000...
-
IBM recently saved $250 million over three years by implementing supply chain software that reduced the cost of components used in its manufacture of computers. If we assume that the savings occurred...
-
List at least five reasons that inventory is a complex accounting and auditing area.
-
Prove that the weights in Eq. (11.18) add up to one. (Hint: Use the geometric series.)
-
Dan Majerle Company sells 10% bonds having a maturity value of $2,000,000 for $1,855,816. The bonds are dated January 1, 2008, and mature January 1, 2013. Interest is payable annually on January 1....
-
9:017 Safari Carlege of Department of Finance wwuction to and ACTU Du dute A) Use the following partial work sheet from Matthews Lanes to prepares Income statement, statement of changes in equity and...
-
Acetonitrile, CH 3 CN, is an important solvent. The chemical is normally available as a by-product of the manufacture of acrylonitrile, CH 2 =CHCN, the building block of polyacrylonitrile, a widely...
-
Chlorine atoms are formed by photochemical reactions of chlorofluorocarbons in the upper atmosphere. Using the average bond energy of the CCl bond in Table 8.8, calculate the wavelength of radiation...
-
A recent college graduate is planning to take the first three actuarial examinations in the coming summer. She will take the first actuarial exam in June. If she passes that exam, then she will take...
-
First, for this case study, define the ethical dilemma facing "John". Second, isn't the collectability of an account ultimately based on opinion? If so , how does that play in the ethical dilemma...
-
Does the game have a dominant-strategy equilibrium? If so, what is it and why is it that? If not, why not?
-
Absorption linewidth for an absorbing atomic transition. Consider the curves of power transmission T(w) = exp[-2am(w)L] through an atomic medium with a lorentzian resonant transition, plotted versus...
-
EXAMPLE 05.04 Z Write the force and the couple in the vector form (with rectangular/Cartesian components). Use C = 180 N-m and P = 500 N O INDIVIDUAL Submission (IS12) D x 400 mm B C 300 mm A 400 mm...
-
1.XYZ Corporation budgets factory overhead cost of P500,000 for the coming year. Compute for the overhead cost applied to the job. The following data are available: Budgeted annual overhead for...
-
Find bases for the eigenspaces of the matrices in Exercise 7. Exercise 7 Find the characteristic equations of the following matrices: 0001 2120 0010 0100
-
1. Which of the four major types of information systems do you think is the most valuable to an organization? 2. How do you critically associate the ideas of business agility and business efficiency...
-
Consider the following circuit where the combinational circuit is represented by COMB and clock skew is represented by t skew . Given the following parameters: FF setup time = 20 ns FF hold time = 10...
-
Consider the following circuit where the combinational circuit is represented by COMB and clock skew is represented by tskew. Given the following parameters: FF setup time = 10 ns FF hold time = 2 ns...
-
A Mealy sequential machine has the following state table: Complete the following timing diagram. Clearly mark on the diagram the times at which you should read the values of Z. All state changes...
-
Incorrect answer iconYour answer is incorrect. On January 1, 2021, Crane Corporation had 1,030,000 shares of common stock outstanding. On March 1, the corporation issued 120,000 new shares to raise...
-
What is the net book value of a non current asset represent? Does the network value represent the market value of the asset?
-
Question 8 (3 points) Which of the following is a "preventative control" (versus a detective control)? Taking periodic inventory. Month-end bank reconciliations. Periodically checking customer...

Study smarter with the SolutionInn App


