The metabolic disorder diabetes causes a buildup of acetone, CH 3 COCH 3 , in the blood.
Question:
The metabolic disorder diabetes causes a buildup of acetone, CH3COCH3, in the blood. Acetone, a volatile compound, is exhaled, giving the breath of untreated diabetics a distinctive odor. The acetone is produced by a breakdown of fats in a series of reactions. The equation for the last step, the breakdown of acetoacetic acid to give acetone and CO2, is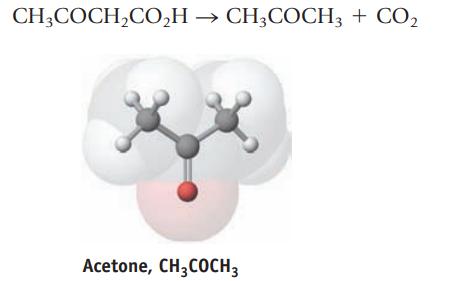
What mass of acetone can be produced from 125 mg of acetoacetic acid?
Transcribed Image Text:
CH3COCH₂CO₂H → CH3COCH3 + CO₂ Acetone, CH3COCH 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The balanced chemical equation for the breakdown of acetoacet...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
A uniform beam of mass m is inclined at an angle θ to the horizontal. Its upper end produces a ninety degree bend in a very rough rope tied to a wall, and its lower end rests on a rough floor...
-
Commercial sodium hydrosulfite is 90.1% Na 2 S 2 O 4 . The sequence of reactions used to prepare the compound is (a) What mass of pure Na 2 S 2 O 4 can be prepared from 125 kg of Zn, 500. g of SO 2 ,...
-
The carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide. 2LiOH(s) + CO2(g) Li2CO3(s) + H2O(l ) Estimate the grams of lithium...
-
If you were the lead analyst for LGM, how would you determine the requirements? Be specific in your answer. List several questions you need answered. Artif. Intell. Med., 56 (3) pp. 137-156, Link:...
-
1. Set up 3-sigma control limits with the given data. 2. Is the process in control? Why? 3. Based on your analysis, do you think the problem is the new computer system or something else? 4. What...
-
Solve parts b and d of Problem 2.67 assuming that the free end of the rope is attached to the crate. Problem 2.67: A 280-kg crate is supported by several rope-and-pulley arrangements as shown....
-
The following stocks make up the Dow Jones Industrial Average (Barrons, March 23, 2009). 1. 3M 11. Disney 21. McDonalds 2. AT&T 12. DuPont 22. Merck 3. Alcoa 13. ExxonMobil
-
Average Rate of Return Method, Net Present Value Method, and Analysis the capital investment committee of Safety Haul Trucking Inc. is considering two investment projects. The estimated income from...
-
Use yahoo finance to find information for the common stock of Pfizer Corporation. Pfizer Inc. develops, manufactures, and sells healthcare products worldwide. It offers medicines and vaccines in...
-
Suppose 16.04 g of benzene, C 6 H 6 , is burned in oxygen. (a) What are the products of the reaction? (b) Write a balanced equation for the reaction. (c) What mass of O 2 , in grams, is required for...
-
The nitrite ion is involved in the biochemical nitrogen cycle. You can determine the nitrite ion content of a sample using spectrophotometry by first using several organic compounds to form a colored...
-
Ethene, C 2 H 4 , is unstable with respect to decomposition to the elements ( f H = 52.47 kJ/mol). It is also unstable with respect to polymerization to polyethylene. Nevertheless, samples of ethane...
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob ODonnell, a local merchant, to contribute the capital to form a partnership....
-
One of the main purposes of evaluation research is: a. reexamining previously collected data. b. monitoring and improving programs. c. generating rich descriptions of individual perspectives. d....
-
10.13 Sweetlip Ltd and Warehou Ltd are two family-owned flax-producing companies in New Zealand. Sweetlip Ltd is owned by the Wood family and the Bradbury family owns Warehou Ltd. The Wood family has...
-
distribution that is skewed to the right instead of being normally distributed. Assume that we collect a random sample of annual incomes of 50 statistics students. Can the distribution of incomes in...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
Consider Wal-Mart, the world's largest retailer. Classify the following items as an Asset (A), a Liability (L), or an Owners' Equity (E) for Wal-Mart (pp. 10-11): ___ a. Accounts payable ___ b....
-
Planning: Creating an Audience Profile; Collaboration: Team Projects. Compare the Facebook pages of three companies in the same industry. Analyze the content on all available tabs. What can you...
-
Propose an efficient synthesis for the following transformation. Br
-
The mean solar flux at the Earths surface is ~2.00 J cm 2 min 1 . In a non-focusing solar collector, the temperature reaches a value of 79.5C. A heat engine is operated using the collector as the hot...
-
Propose a plausible mechanism for the following transformation. C CI
-
(i) (Click the icon is view the (Assume bonds payable are a ) nearest whole dollar.) More info a. Issuance of the bonds on January 1,2018. b. Payment of interest and amortizaton on dune 30,2018. c....
-
The Gilmore Insurance Group earned a profit of $10,000 in its first month of business. Then, its profit increased by 3% each month for the next two years. What is the total profit that the Gilmore...
-
BluStar Company has two service departments, Administration and Accounting, and two operating departments, Domestic and International. Administration costs are allocated on the basis of employees,...

Study smarter with the SolutionInn App


