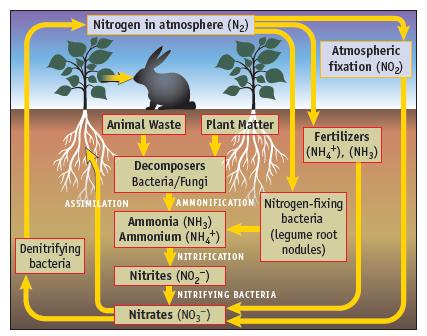
The nitrogen cycle (Figure 20.1) shows the oxidation of NH 4 + , first to NO 2
Question:
The nitrogen cycle (Figure 20.1) shows the oxidation of NH4+, first to NO2− and then the subsequent oxidation of NO2− to NO3−. Write balanced equations for each of these half-reactions (in acid solution).
Data given in Figure 20.1
Transcribed Image Text:
Nitrogen in atmosphere (N₂) Denitrifying bacteria Animal Waste Plant Matter ASSIMILATION Decomposers Bacteria/Fungi Ammonia (NH3) Ammonium (NH4+) AMMONIFICATION Nitrogen-fixing bacteria NITRIFICATION Nitrites (NO₂) Atmospheric fixation (NO₂) NITRIFYING BACTERIA Nitrates (NO3) Fertilizers (NH,*), (NH3) (legume root nodules)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The following are balanced equations for the oxidation of NH4 to NO2 and the oxidation of NO2 to NO3 ...View the full answer

Answered By

Sidharth Jain
My name is Sidharth. I completed engineering from National Institute of Technology Durgapur which is one of the top college in India. I am currently working as an Maths Faculty in one of the biggest IITJEE institute in India. Due to my passion in teaching and Maths, I came to this field. I've been teaching for almost 3 years.
Apart from it I also worked as an Expert Answerer on Chegg.com. I have many clients from USA to whom I teach online and help them in their assignments. I worked on many online classes on mymathlab and webassign. I guarantee for grade 'A'.
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Write balanced equations for each of the following reactions (some of these are analogous to reactions shown in the chapter). (a) Aluminum metal reacts with acids to form hydrogen gas. (b) Steam...
-
Write balanced equations for the reactions described in Table 18.13 for the production of Bi and Sb. Table 18.13 Element Nitrogen Phosphorus Electronegativity Source Method of Preparation 3.0 2.2 Air...
-
Write balanced equations for each of the following. a. Solid calcium fluoride is heated with sulfuric acid to give hydrogen fluoride vapor. b. Solid potassium chlorate is carefully heated to yield...
-
Can you give us a few Google AdWords Ad Extensions names that you know?
-
Integrated Technologies Inc. is considering the purchase of automated machinery that is expected to have a useful life of four years and no residual value. The average rate of return on the average...
-
1. When auditing prepaid insurance, an auditor discovers that the original insurance policy on a key piece of manufacturing equipment is not avail-able for inspection. The policys absence most likely...
-
The following table shows quarterly demand data for 3 consecutive years: Quarter Year I II III IV 2008 21 27 41 13 2009 19 32 42 12 2010 22 33 38 10 Choose smoothing coefficients and apply...
-
A condensed balance sheet for a partnership to be liquidated is as follows: The profit and loss percentages for Partners A, B, and C are 50%, 30%, and 20%, respectively. For each of the following...
-
In the Assembly Department of Ayayai Company, budgeted and actual manufacturing overhead costs for the month of April 2 0 2 7 were as follows. \ table [ [ , Budget,Actual ] , [ Indirect materials,$ 1...
-
Refer to the figure below and Figure 20.25, which show the fraction of species in solution [alpha ()] as a function of pH. The following questions are in regard to the equilibria involved in an...
-
The refrigerating liquids in air conditioners and refrigerators are largely chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs). Among the latter family of compounds is the refrigerant...
-
Calculating Interest Expense you receive a credit card application from Shady Banks Savings and Loan offering an introductory rate of 2.5 percent per year, compounded monthly for the first six...
-
Find the limit analytically. -7x2+5x-10 lim 0 9x+13x+11 Find the limit analytically. lim +80 4x-13 5x+6x-11
-
Write a recursive function for the running time T(n) of the function given below. Prove using the iterative method that T(n) = (n). function( int n) { if(n=1) return; for(int i = 1; i
-
1. A T-shaped beam with an overhang is supported and loaded as shown in Fig. 1. Draw shear force diagram and calculate (a) the shear stress at a point D, 2 m from support A and 25 mm from the top of...
-
Question 1: Write Specific Case to brief is Flying Fish Bikes, Inc. v. Giant Bicycle, Inc., 181 F.Supp.3d 957? Grading Rubric for Case Brief Written Case Brief 3 (Exceeds Identification - Heading...
-
A local gym is looking in to purchasing more exercise equipment and runs a survey to find out the preference in exercise equipment amongst their members. They categorize the members based on how...
-
Find An if n is a positive integer and 013 121 310
-
Cobb Manufacturing Company uses a process cost system and average costing. The following production data is for the month of June 2011. Production Costs Work in process, beginning of the month:...
-
Derive the state transition table and D flip-flop input equations for a counter that counts from 1 to 6 (and back to 1 and continues).
-
Reduce the following state table to a minimum number of states. Present State Next State Output X = 1 X = 0 X= 1 B A D D G D
-
A Mealy sequential circuit is implemented using the circuit shown in Problem 1.26. Assume that if the input X changes, it changes at the same time as the falling edge of the clock. (a) Complete the...
-
Gross margin can be found on single step income statement. TRUE FALSE GAAP requires companies to disclose the sales returns and allowance on their Income Statement. TRUE FALSE
-
) Identify how each of the following items is shown on the statement of cash flows. Identify each as operating (O), investing (I), financing (F), or non-cash investing and financing (N). Item (O),...
-
1. Skolnick Corporation has provided the following information: Cost per Unit Cost per Period Direct materials $ 5.50 Direct labor $ 3.30 Variable manufacturing overhead $ 2.10 Fixed manufacturing...

Study smarter with the SolutionInn App


