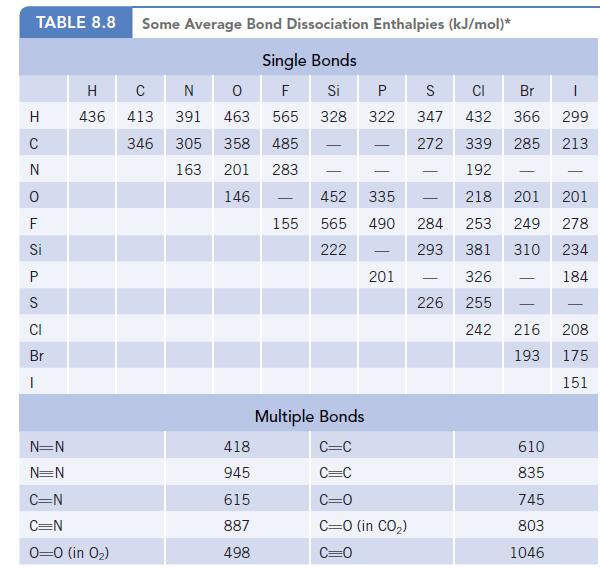
Use the bond dissociation enthalpies in Table 8.8 to estimate the enthalpy change for the decomposition of
Question:
Use the bond dissociation enthalpies in Table 8.8 to estimate the enthalpy change for the decomposition of urea to hydrazine, H2N—NH2, and carbon monoxide. (Assume all compounds are in the gas phase.)
Data given in Table 8.8
Transcribed Image Text:
TABLE 8.8 Some Average Bond Dissociation Enthalpies (kJ/mol)* Single Bonds F Si 328 H C N 0 F Si P S CI Br B H с N 0 436 413 391 463 346 305 358 163 201 146 N=N N=N C=N C=N 0-0 (in 0₂) 418 945 615 887 498 565 485 283 155 - P 322 - 452 335 565 490 284 222 293 201 S CI Br 347 432 366 272 339 285 192 218 201 253 249 381 310 326 255 242 Multiple Bonds C=C C=C C=0 C=0 (in CO₂) 226 - - 216 193 610 835 745 803 1046 1 299 213 201 278 234 184 - 208 175 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
H H O H H urea H H H H hydrazine carbon monox...View the full answer

Answered By

Mishark muli
Having any assignments and any other research related work? worry less for I am ready to help you with any task. I am quality oriented and dedicated always to produce good and presentable work for the client once he/she entrusts me with their work. i guarantee also non plagiarized work and well researched work to give you straight As in all your units.Feel free to consult me for any help and you will never regret
4.70+
11+ Reviews
37+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Phosgene, Cl 2 CO, is a highly toxic gas that was used as a weapon in World War I. Using the bond dissociation enthalpies in Table 8.8, estimate the enthalpy change for the reaction of carbon...
-
The equation for the combustion of gaseous methanol is (a) Using the bond dissociation enthalpies in Table 8.8, estimate the enthalpy change for this reaction. What is the enthalpy of combustion of...
-
Hydrogenation reactions, which involve the addition of H 2 to a molecule, are widely used in industry to transform one compound into another. For example, 1-butene (C 4 H 8 ) is converted to butane...
-
Obtain the transfer functions X(s)/F(s) and Y(s)/F(s) for the following model: 3x = y y = f(t) -3y- 15x
-
Why is an accurate and complete requirements definition especially critical when using the SDLC waterfall approach?
-
On January 1, 2010, the controller of Gardeneer Tools Inc. is planning capital expenditures for the years 20102013. The controller interviewed several Gardeneer executives to collect the necessary...
-
20.4 Samantha bought a house for 37,500 on 1 August 1988 and occupied the house as her principal private residence. On 1 June 1990 she began to use one-fifth of the house for business purposes....
-
Elliot & Hesse Inc. manufactures ergonomic devices for computer users. Some of its more popular products include glare screens (for computer monitors), keyboard stands with wrist rests, and carousels...
-
Required information The Foundational 15 (Static) (LO1-1, LO1-2, LO1-3, LO1-4, LO1-5, LO1-6] [The following information applies to the questions displayed below.) Martinez Company's relevant range of...
-
Dihydroxyacetone is a component of quick tanning lotions. (It reacts with the amino acids in the upper layer of skin and colors them brown in a reaction similar to that occurring when food is browned...
-
The molecule shown here, 2-furylmethanethiol, is responsible for the aroma of coffee: (a) What are the formal charges on the S and O atoms? (b) Give approximate values of angles 1, 2, and 3. (c)...
-
What does the evidence on the effects of the partial U.S. ban on cigarette advertising suggest about the relative effectiveness of cigarette advertising versus antismoking advertising?
-
Were you surprised by the results? Do you agree with the results? How can you use this knowledge of your personal biases to inform your management strategies? How can the identified biases impact...
-
what ways do existing power structures perpetuate social stratification, and what are the socio-political ramifications of these dynamics ?
-
How do feedback loops and reflective practices contribute to continuous improvement and the refinement of teamwork dynamics over time ? Explain
-
What did the NFL do to create much needed visibility for corporate sponsors during the football season when fans were not allowed at games due to the virus?
-
The Haines Corporation shows the following financial data for 20X1 and 20X2: Sales Cost of goods sold Selling & administrative expense Gross profit Operating profit Interest expense Income before...
-
Test two integrated circuits. In each test, the probability of rejecting the circuit is p, independent of the other test. Let X be the number of rejects (either 0 or 1) in the first test and let Y be...
-
How does health insurance risk differ from other types of insurance risk (e.g., automobile or homeowners insurance)? What is the difference between cost sharing and cost shifting? Is retiree health...
-
Discuss why a quantum mechanical particle in a box has zero point energy in terms of its wavelength.
-
How does an expectation value for an observable differ from an average of all possible eigenvalues?
-
We set the potential energy in the particle in the box equal to zero and justified it by saying that there is no absolute scale for potential energy. Is this also true for kinetic energy?
-
The following are selected 2020 transactions of Pronghorn Corporation. Sept. 1 Purchased inventory from Encino Company on account for $47,400. Pronghorn records purchases gross and uses a periodic...
-
At January 1, 2021, Caf Med leased restaurant equipment from Crescent Corporation under a nine-year lease agreement. The lease agreement specifies annual payments of $22,000 beginning January 1,...
-
Anglin Company, a manufacturing firm, has supplied the following information from its accounting records for the last calendar year: Direct labor cost $494,770 375,000 7,850 Purchases of direct...

Study smarter with the SolutionInn App


