Phosgene, Cl 2 CO, is a highly toxic gas that was used as a weapon in World
Question:
Phosgene, Cl2CO, is a highly toxic gas that was used as a weapon in World War I. Using the bond dissociation enthalpies in Table 8.8, estimate the enthalpy change for the reaction of carbon monoxide and chlorine to produce phosgene.![]()
Data given in table 8.8
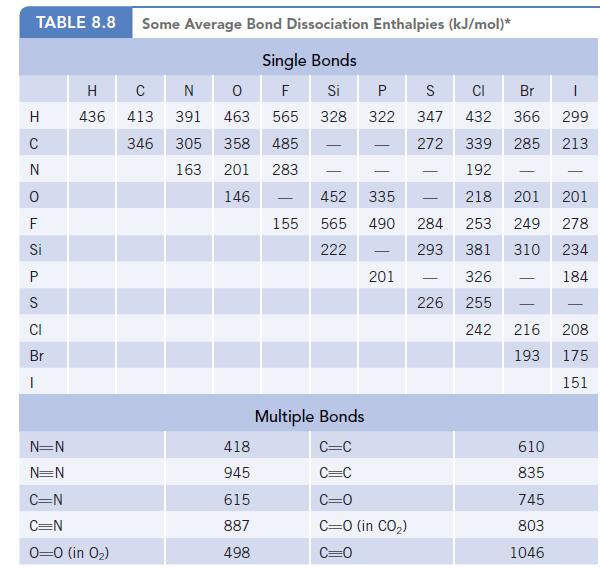
Transcribed Image Text:
CO(g) + Cl₂(g) → Cl₂CO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 16% (6 reviews)
CO Cl CICO AHrxn AHbonds brokenAHbonds ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Phosgene is highly toxic and was used as a chemical weapon in World War I. It is also a synthetic precursor used in the production of many plastics. (a) When vapors of phosgene are inhaled, the...
-
Use the bond dissociation enthalpies in Table 8.8 to estimate the enthalpy change for the decomposition of urea to hydrazine, H 2 NNH 2 , and carbon monoxide. (Assume all compounds are in the gas...
-
Phosgene (CC1 2 O) is a colorless gas that was used as an agent of chemical warfare in World War I. It has the odor of newly mown hay (which is a good warning if you know the smell of newly mown...
-
Design a suitable angle section to carry a factored tensile force 210 kN assuming a single row of M20 bolts. The yield strength and ultimate strength of the material is 250 MPa and 410 MPa,...
-
Web services have been called a second wave of net-centric computing that will have broad implications for software development approaches in the future. Develop an argument to support or refute this...
-
Natalie Koebel spent much of her childhood learning the art of cookie-making from her grandmother. They passed many happy hours mastering every type of cookie imaginable and later creating new...
-
17.5 In June 2000, Gillian was given a chargeable asset with a market value at that time of 6,000. In November 2008 she made a successful claim to the effect that the asset now had a negligible value...
-
On August 1, 2016, Bill Hudson established Heritage Realty, which completed the following transactions during the month: a. Bill Hudson transferred cash from a personal bank account to an account to...
-
PROBLEM 12-7A Prepare a Statement of Cash Flows LO 12-1, LOI2-2) Comparative financial statements for Weaver Company follow: Weaver Company Comparative Balance Sheet December 31, 2015 and 2014 2015...
-
Consider the carbonoxygen bond in formaldehyde (CH 2 O) and carbon monoxide (CO). In which molecule is the CO bond shorter? In which molecule is the CO bond stronger?
-
Methanol can be made by partial oxidation of methane using O 2 in the presence of a catalyst: Use bond dissociation enthalpies to estimate the enthalpy change for this reaction. Compare the value...
-
Dagmar Company budgeted the following amounts: Variable costs of production: Direct materials ....... 3 pounds @ 0.80 per pound Direct labor ........ 0.5 hr. @ $12.00 per hour Variable overhead...
-
Do we drive technology, or does technology drive us? If technology drives us, what are the risks? The other side of the coin would be that we are able to stay ahead of technological transformations....
-
How do you explain the differences between the two analyses and what are the implications of using the BCG matrix in practice?
-
How do leadership styles, such as transformational leadership, shared leadership, and servant leadership, impact team dynamics, member motivation, and overall team effectiveness ?
-
How do these relevant legal principles apply: Duty of care Duty of obedience Duty of loyalty Shareholder Derivative suit Piercing the corporate veil...
-
what will you do as a hotel manager if a customer complained about bad service they received?
-
An instant lottery ticket consists of a collection of boxes covered with gray wax. For a subset of the boxes, the gray wax hides a special mark. If a player scratches off the correct number of the...
-
Prove the formula for (d/dx)(cos-1x) by the same method as for (d/dx)(sin-1x).
-
Semiconductors can become conductive if their temperature is raised sufficiently to populate the (empty) conduction band from the highest filled levels in the valence band. The ratio of the...
-
For the Ï-network of β-carotene modeled using the particle in the box, the position-dependent probability density of finding 1 of the 22 electrons is given by The quantum number n in...
-
Calculate the energy levels of the -network in hexatriene, C 6 H 8 , using the particle in the box model. To calculate the box length, assume that the molecule is linear and use the values 135 and...
-
Periodic Inventory Using FIFO, LIFO, and Weighted Average Cost Methods The units of an item available for sale during the year were as follows: Jan. 1 18 units at $26 $468 Inventory Purchase Purchase...
-
Engineering Economics; Could you please solve all 3 questions, I'll give you a thumbs up, I have 1 hour left, thank you in advance sir.. 2.(20P) A company is planning to buy a minibus for 380 000 TL...
-
1 Anu Company-Solving for unknowns Anu Company produces chocolate almond bars. Each bar sells for 4. The variable costs for each bar (sugar. chocolate, almonds, wrapper, labour, and so on) amount to...

Study smarter with the SolutionInn App


