Using enthalpy of formation data in Appendix L, determine whether the decomposition of NH 4 NO 3
Question:
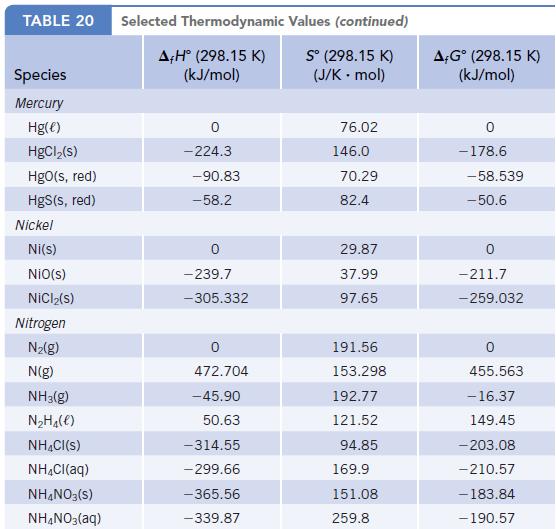
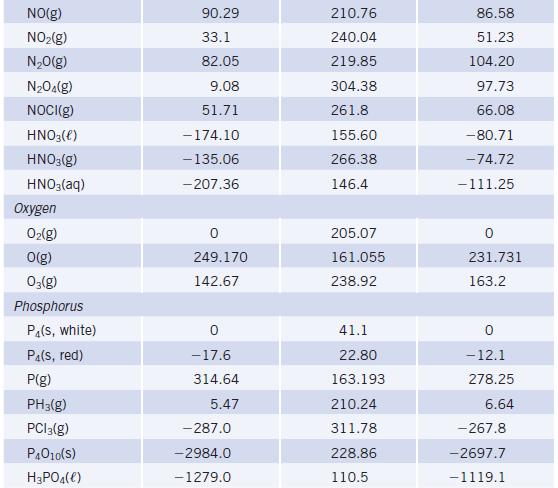
Using enthalpy of formation data in Appendix L, determine whether the decomposition of NH4NO3(s) to give N2O(g) and H2O(g) is endothermic or exothermic.
Data given in Appendix L
Transcribed Image Text:
TABLE 20 Species Mercury Hg(e) HgCl₂(s) HgO(s, red) HgS(s, red) Nickel Ni(s) NiO(s) NiCl₂(s) Nitrogen N₂(g) N(g) NH3(g) N₂H₁(e) NH4Cl(S) NH₂Cl(aq) NH4NO3(S) NH4NO3(aq) Selected Thermodynamic A+Hº (298.15 K) (kJ/mol) 0 -224.3 -90.83 -58.2 -239.7 -305.332 472.704 -45.90 50.63 -314.55 - 299.66 -365.56 -339.87 Values (continued) S° (298.15 K) (J/K - mol) 76.02 146.0 70.29 82.4 29.87 37.99 97.65 191.56 153.298 192.77 121.52 94.85 169.9 151.08 259.8 A-Gº (298.15 K) (kJ/mol) 0 -178.6 -58.539 -50.6 -211.7 -259.032 455.563 -16.37 149.45 -203.08 -210.57 -183.84 - 190.57
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
NHNO 30 NO 2HO AH for NH NO 36556 KJmol 300 AH f...View the full answer

Answered By

Chiranjib Thakur
I have no tutoring experience yet, but I can share my skills and knowledge gained from my education and work experiences. I have been a CPA since 2012 with 6 years of work experience in internal auditing and 4 years of work experience in accounting at the supervisory level.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Accordingly, although ISAs do not impose responsibilities on management and those charged with governance and do not override laws and regulations that govern their responsibilities, ISAs are...
-
Use data in Appendix L to calculate the enthalpy and free energy change for the reaction 2 NO 2 (g) N 2 O 4 (g) Is this reaction exothermic or endothermic? Is the reaction product- or...
-
Use data in Appendix L to calculate the enthalpy and free energy change for the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g). Is this reaction exothermic or endothermic? Is the reaction product- or...
-
On December 31, 2021, Tiny Tims Tech, Inc. a private company who follows ASPE, leased a colour copier from Cory Copy Corporation at a price of $479,079. The lease agreement specifies annual payments...
-
Colleges and universities have a variety of internal and external customers. Use a team of three or four individuals to answer the following: a. Who are three internal and two external customers of a...
-
Multiple Choice 1. An independent audit aids in the communication of economic data because the audit a. Confirms the exact accuracy of managements financial representations. b. Lends credibility to...
-
A Problem in Decision-Making. The Middle-Atlantic Territory of the Flick Company has produced results for the past three years which average as follows: Sales Cost of goods sold-standard Gross profit...
-
The food services division of Cedar River Amusement Park Inc. is studying the amount that families who visit the amusement park spend per day on food and drink. A sample of 40 families who visited...
-
2007 2006 2005 Sales 61,471 57,878 51,271 Credit Card Revenues 1,896 1,612 1,349 Total Revenues 63,367 59,490 52,620 Cost of Sales 41,895 39,399 34,927 Selling, General and Administrative Expenses...
-
The chemistry of gallium: (a) Gallium hydroxide, like aluminum hydroxide, is amphoteric. Write a balanced equation to show how this hydroxide can dissolve in both HCl(aq) and NaOH(aq). (b) Gallium...
-
When palladium metal is exposed to H 2 gas, the metal become brittle because H 2 molecules dissociate and H atoms fill some of the octahedral holes in the face-centered cubic lattice. To find the...
-
In the figure below, both circles are centered around X. The length of XY is 2 units and the length of XZ is 6 units. If the smaller circle is cut out of the larger circle, how much of the area, in...
-
Marc Goudreau, administrator of Clearwater Hospital, was puzzled by the prior month's reports. "Every month, it's anyone's guess whether the lab will show a profit or a loss. Perhaps the only answer...
-
A system consisting of a gas consisting of O2 (32 Da), H2 (2 Da), and Ar (40 Da) molecules and a billiard ball is at some temperature . Relative to O2, the billiard ball is 1.0 E+26 times as massive...
-
Low Desert Pottery works makes a variety of pottery products that it sells to retailers. The company uses a job-order costing system in which departmental predetermined overhead rates are used to...
-
ASSESSMENT CPCCBC5002A Monitor costing systems on medium rise building and construction projects Please provide answer to Part 2 - Monitor expenditure for a medium-rise project as per below...
-
Questions 6-8 refer to the same problem A sinusoidal wave with wavelength 2 m and amplitude 5 mm is traveling along the x axis. The wave is traveling in the -x direction at a speed of 2m/s At t = Os,...
-
Let T1: P1 P2 be the linear transformation defined by T1(c0 + c1x) = 2c0 - 3c1x and let T2: P2 P3 be the linear transformation defined by T2(c0 + c1x + c2x2) = 3c0x + 3c1x2 + 3c2x3 Let B = (1, x),...
-
The Home Depot is the leading retailer in the home improvement industry and one of the 10largest retailers in the United States. The company included the following on its January 29, 2012, balance...
-
A candle flame is 18.0 cm in front of a thin positive lens. Its image appears three times farther away from the lens than if the same candle were on a very distant mountain. Determine the lenss focal...
-
What must the focal length of a thin negative lens be for it to form a virtual image 50 cm away (measured from the lens) of an ant located 100 cm away (measured from the lens)? Given (just as a...
-
An LED is on the central axis 30.0 cm in front of a thin lens. The resulting image, which is virtual, is 10.0 cm from the lens. Determine the focal length of the lens. Using Table 5.3, explain why...
-
Spencer Co. has a $360 petty cash fund. At the end of the first month the accumulated receipts represent $59 for delivery expenses, $191 for merchandise inventory, and $28 for miscellaneous expense....
-
A July sales forecast projects that 10,000 units are going to be sold at a price of $11.50 per unit. Management forecasts 4% growth in sales each month. Total August sales are anticipated to be:...
-
What provides the user with the highest level of assurance that the financial statements are free from material misstatement and/or that the financial statements do not require a material...

Study smarter with the SolutionInn App


