Verify that the atomic weight of lithium is 6.94, given the following information: 6 Li, mass =
Question:
Verify that the atomic weight of lithium is 6.94, given the following information:
6Li, mass = 6.015121 u; percent abundance = 7.50%
7Li, mass = 7.016003 u; percent abundance = 92.50%

Transcribed Image Text:
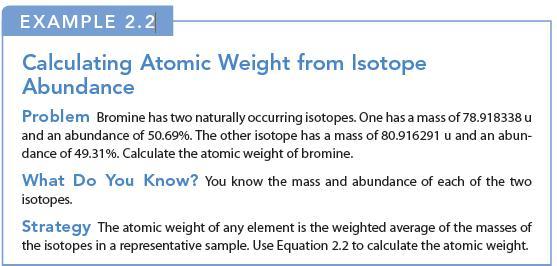
EXAMPLE 2.2 Calculating Atomic Weight from Isotope Abundance Problem Bromine has two naturally occurring isotopes. One has a mass of 78.918338 u and an abundance of 50.69%. The other isotope has a mass of 80.916291 u and an abun- dance of 49.31%. Calculate the atomic weight of bromine. What Do You Know? You know the mass and abundance of each of the two isotopes. Strategy The atomic weight of any element is the weighted average of the masses of the isotopes in a representative sample. Use Equation 2.2 to calculate the atomic weight.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Here to two isotope of Li is given Each natural abundance is different both mix and g...View the full answer

Answered By

Elias Gichuru
am devoted to my work and dedicated in helping my clients accomplish their goals and objectives,providing the best for all tasks assigned to me as a freelancer,providing high quality work that yields high scores.promise to serve them earnestly and help them achieve their goals.i have the needed expertise,knowledge and experience to handle their tasks.
4.80+
325+ Reviews
859+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Verify that the atomic weight of magnesium is 24.31, given the following information: 24 Mg, mass = 23.985042 u; percent abundance = 78.99% 25 Mg, mass = 24.985837 u; percent abundance = 10.00% 26...
-
Thallium has two stable isotopes, 203 Tl and 205 Tl. Knowing that the atomic weight of thallium is 204.4, which isotope is the more abundant of the two? EXAMPLE 2.2 Calculating Atomic Weight from...
-
Europium has two stable isotopes, 151 Eu and 153 Eu, with masses of 150.9197 u and 152.9212 u, respectively. Calculate the percent abundances of these isotopes of europium. EXAMPLE 2.2 Calculating...
-
Sherman Co. began operations on January 1, 2015, and completed several transactions during 2015 and 2016 that involved sales on credit, accounts receivable collections, and bad debts. These...
-
Describe seven principles for a user-centered interface design.
-
1. Determine whether each of the following is best described as a fixed, variable, or mixer cost with respect to product units. 2. Following are five graphs representing various cost behaviors. Thank...
-
(Analysis of Alternatives) The Black Knights Inc., a manufacturer of high-sugar, low-sodium, low-cholesterol TV dinners, would like to increase its market share in the Sunbelt. In order to do so,...
-
Clear channel, an owner of multiple radio stations with the Top 40 format, recently bought rock concert promoter Live Nation. How would this affect prices for concert tickets or rates for radio...
-
Subject : Advanced Accounting PROBLEM 16. Statement of Affairs and Deficiency Account LOT Phot Chas filed ukupy petites cas December 31, 20 Tel Cud $ 2.500 Na Race Am Receivable et 76.00 mories...
-
Gallium has two naturally occurring isotopes, 69 Ga and 71 Ga, with masses of 68.9257 u and 70.9249 u, respectively. Calculate the percent abundances of these isotopes of gallium. EXAMPLE 2.2...
-
Name and describe the composition of the three hydrogen isotopes.
-
Use improved customer satisfaction as a marketing tool.
-
Scatterplot. In Exercises 5-8, use the sample data to construct a scatterplot. Use the first variable for the x-axis. Based on the scatterplot, what do you conclude about a linear correlation? Pulse...
-
Given two fair six sided dice and a standard deck of 52 playing cards, calculate the probability of a rolling a sum of 7 or 11 and drawing three cards in which at least one is a face card.
-
z Scores. In Exercises 5-8, express all z scores with two decimal places. 5. Diastolic Blood Pressure of Females For the diastolic blood pressure measurements of females listed in Data Set 1 "Body...
-
A jury of 12 is to be created from a pool of 20 men and 10 women. What is the probability that all 12 on the jury will be men?
-
Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit sales in 2020 General Ledger...
-
a. Some banks have offered their customers an unusual type of time deposit. The deposit does not pay any interest if the market falls, but instead the depositor receives a proportion of any rise in...
-
(a) Use integration by parts to show that (b) If f and g are inverse functions and f' is continuous, prove that (c) In the case where f and t are positive functions and b > a > 0, draw a diagram to...
-
Rewrite the van der Waals equation using the molar volume rather than V and n.
-
Propose a plausible synthesis for each of the following transformations: (a) (b) (c) (d)
-
Calculate w for the adiabatic expansion of 2.50 mol of an ideal gas at an initial pressure of 2.25 bar from an initial temperature of 450. K to a final temperature of 300. K. Write an expression for...
-
The balance sheets for Federer Sports Apparel for 2022 and 2021 are presented below. 2. Prepare a horizontal analysis for 2022 using 2021 as the base year. (Note: If the percentage increase or...
-
Bergo Bay's accounting system generated the following account balances on December 3 1 . The company's manager knows something is wrong with this list of balances because it does not show any balance...
-
(Compound annuity) What is the accumulated sum of each of the following streams of payments? a. $500 a year for 8 years compounded annually at 11 percent. b. $104 a year for 8 years compounded...

Study smarter with the SolutionInn App


