Verify that the atomic weight of magnesium is 24.31, given the following information: 24 Mg, mass =
Question:
Verify that the atomic weight of magnesium is 24.31, given the following information:
24Mg, mass = 23.985042 u; percent abundance = 78.99%
25Mg, mass = 24.985837 u; percent abundance = 10.00%
26Mg, mass = 25.982593 u; percent abundance = 11.01%

Transcribed Image Text:
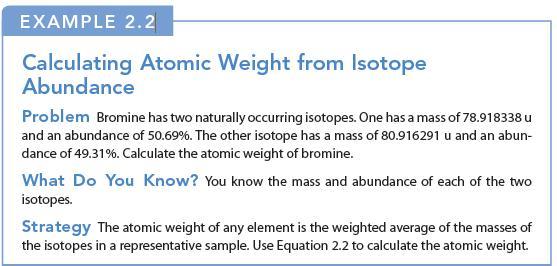
EXAMPLE 2.2 Calculating Atomic Weight from Isotope Abundance Problem Bromine has two naturally occurring isotopes. One has a mass of 78.918338 u and an abundance of 50.69%. The other isotope has a mass of 80.916291 u and an abun- dance of 49.31%. Calculate the atomic weight of bromine. What Do You Know? You know the mass and abundance of each of the two isotopes. Strategy The atomic weight of any element is the weighted average of the masses of the isotopes in a representative sample. Use Equation 2.2 to calculate the atomic weight.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
To calculate the atomic weight of magnesium Mg given the isotopic data pro...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Verify that the atomic weight of lithium is 6.94, given the following information: 6 Li, mass = 6.015121 u; percent abundance = 7.50% 7 Li, mass = 7.016003 u; percent abundance = 92.50% EXAMPLE 2.2...
-
Thallium has two stable isotopes, 203 Tl and 205 Tl. Knowing that the atomic weight of thallium is 204.4, which isotope is the more abundant of the two? EXAMPLE 2.2 Calculating Atomic Weight from...
-
Europium has two stable isotopes, 151 Eu and 153 Eu, with masses of 150.9197 u and 152.9212 u, respectively. Calculate the percent abundances of these isotopes of europium. EXAMPLE 2.2 Calculating...
-
The Home Depot, Inc. (HD) operates over 2,200 home improvement retail stores and is a competitor of Lowe's (LOW). The following data (in millions) were adapted from recent financial statements of The...
-
Describe six types of user interface controls, and provide an example of how you could use each type in a data entry screen.
-
At the beginning of the month, you sell short 200 shares of Wells Fargo that are currently selling at $45 per share. One month later you cover your short position at $52 per shares. During the month,...
-
(Computation of Bond Liability) Lance Armstrong Inc. manufactures cycling equipment. Recently the vice president of operations of the company has requested construction of a new plant to meet the...
-
Sierra Company incurs the following costs to produce and sell a single product. During the last year, 25,000 units were produced and 22,000 units were sold. The Finished Goods inventory account at...
-
true or false 17 Acompany estimates the following manufacturing costs at the beginning of the period: direct labor, $117,000, direct materials. $97,500, and factory overhead $29.250. The company's...
-
Gallium has two naturally occurring isotopes, 69 Ga and 71 Ga, with masses of 68.9257 u and 70.9249 u, respectively. Calculate the percent abundances of these isotopes of gallium. EXAMPLE 2.2...
-
Name and describe the composition of the three hydrogen isotopes.
-
Design a MOSFET circuit with the configuration shown in Figure P3.30. The transistor parameters are \(V_{T P}=-0.6 \mathrm{~V}, k_{p}^{\prime}=50 \mu \mathrm{A} / \mathrm{V}^{2}\), and \(\lambda=0\)....
-
Y = AK[1-a R P = QAKa-1[1-a W P = (1 -Q) AKL-a 1= 14 1 -4 Y = C
-
Inferring Transactions from Financial Statements (FSET) Wired.com Inc. is a large e-commerce company, with over $31 billion in revenues for the fiscal year ended December 31, 20X2. For the year ended...
-
Finding Standard Deviation from a Frequency Distribution. In Exercises 37-40, refer to the frequency distribution in the given exercise and compute the standard deviation by using the formula below,...
-
For the past 30 years, the average satisfaction rating for a sushi restaurant has been 3.9 out of 5. If the rating for a sample of 256 people is 4.1 with a standard deviation of 0.5, the critical...
-
Hash collisions occur when more than one item is mapped to the same element in Hash Table's array. What is one way that a Hash Table can handle collisions?
-
After dramatic increases in oil prices in the 1970s, the U.S. government funded several projects to create synthetic oil or natural gas from abundant U.S. supplies of coal and oil shale. Although the...
-
A survey of 70 college freshmen asked whether students planned to take biology, chemistry, or physics during their first year. Use the diagram to answer each question. How many of the surveyed...
-
Why do the z and y components of the velocity not change in the collision depicted in Figure 1.2? Figure 1.2 mvx mvx
-
A mixture of 2.10 10 3 g of O 2 , 3.88 10 -3 mol of N 2 , and 5.25 10 20 molecules of CO are placed into a vessel of volume 5.25 L at 12.5C. a. Calculate the total pressure in the vessel. b....
-
Propose a plausible synthesis for each of the following transformations: (a) (b) (c) (d)
-
Taxpayers may use historical data to determine the recovery period for tax depreciation. True or False True False
-
The manager of Wildhorse, Inc. reviewed the year just completed, hoping to establish a point of comparison for measuring the company's actual performance. Sales volume came in lower than expected, at...
-
On July 1, a company paid the $3,360 premium on a one-year insurance policy with benefits beginning on that date. What will be the insurance expense on the annual income statement for the first year...

Study smarter with the SolutionInn App


