You have a 250.0-mL graduated cylinder containing some water. You drop three marbles with a total mass
Question:
You have a 250.0-mL graduated cylinder containing some water. You drop three marbles with a total mass of 95.2 g into the water. What is the average density of a marble? 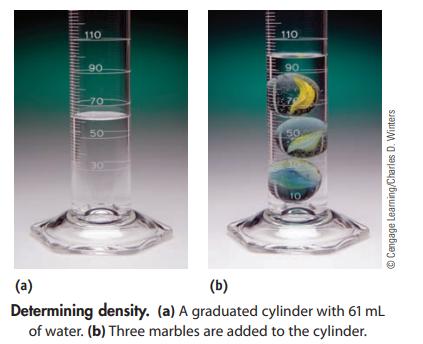
Transcribed Image Text:
110 90 -70 50 30 110 90. 50 10 ⒸCengage Learning/Charles D. Winters (a) (b) Determining density. (a) A graduated cylinder with 61 mL of water. (b) Three marbles are added to the cylinder.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To find the average density of a marble you need to know the change in volume and the change in mass ...View the full answer

Answered By

Anum Naz
Lecturer and researcher with 10+ years of experience teaching courses in both undergraduate and postgraduate levels. Supervised 17 BA theses, 07 MA theses, and 1 Ph.D. dissertations. Edited and co-authored 2 monographs on contemporary trends in political thought. Published over articles in peer-reviewed journals.
4.80+
11+ Reviews
52+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
You set out to determine the density of lead in the laboratory. Using a top loading balance to determine the mass and the water displacement method (Study Question 41) to determine the volume of a...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
Supply chains of the Roman Empire had much in common with modern supply chains. Supply chains then and now require development of good mental models to understand them and keep them working well....
-
In a test on 2000 electric bulbs, it was found that the life of a particular make was normally distributed with an average life of 2040 hos & S.D of bohos Estimate the ne of likely to burn for it...
-
Do employers have a responsibility to alert other employers to an employees wrongdoing by supplying an unfavorable reference? Why or why not? Discuss the conflict between community responsibility and...
-
When shareholders are paid dividends from their investment in a company, it is presented in the statement of cash flows under the heading investing activities." Select one: True False
-
Park Company is considering two alternative investments. The payback period is 3.5 years for investment A and 4 years for investment B. (1) If management relies on the payback period, which...
-
Xographics is a division of a large telecommunications company. Ellen Bohn, the vice president of production, had recently moved to Xographics from a firm where she had been the manager of a large...
-
Income Statement Bobs Bistro produces party-sized hoagie sandwiches. For next year, Bobs Bistro predicts that 50,000 units will be produced with the following total costs: Direct materials ? Direct...
-
Give the symbol for each of the following elements: (a). Barium (b). Titanium (c). Chromium (d). Lead (e). Arsenic (f). Zinc
-
You need a cube of aluminum with a mass of 7.6 g. What must be the length of the cubes edge (in cm)? (The density of aluminum is 2.698 g/cm 3 .)
-
Samples of size n = 6 are collected from a process every hour. After 20 samples have been collected, we calculate x = 20.0 and r / d 2 = 1.4. (a) Calculate trial control limits for X and R charts....
-
1. (20) Let and Dor {abnm or 2n m} = Dand = {a"b" nm and 2n m}. Prove that Dor and Dand are both context-free.
-
Given n samples 1 , 2 , . . . , x 1 ,x 2 ,...,x N drawn independently from a Poisson distribution unknown parameter , find the MLE of . = = 1 MLE = i=1 n x i = = 1 MLE =n i=1 n x i = = 1 MLE = i=1 n...
-
(a)The local police station found that the speed of vehicles travelling around the suburb in the 60 km/hour zone varies uniformly between 55 km/hour and 62 km/hour. What is the probability that the...
-
Consider the following fixed-point iteration: xn+1 = g(xn), where [f(x)] 2 g(x) = x (x + f(x)) f(x)* (a) What is the order of convergence for the method? (e.g. what is p?). Hint: Show that the method...
-
Problem 1. In a study of infant birth weight and maternal factors, the newborn babies were categorized as being either small size for gestational age (N=201) or normal size (N=2089). The following...
-
Design or modify the OUTSTANDING COMPUTER PURCHASE ORDERS REPORT. Refer to the repository entry for the data flow for the elements. This report would be produced for all PURCHASE ORDER records that...
-
Should we separate the debt and equity features of convertible debt? Team 1: Pro separation: Present arguments in favor of separating the debt and equity features of convertible debt. Team 2: Against...
-
The specific rotation of vitamin C (using the D line of sodium, at 20C) is +24. Predict what the observed rotation would be for a solution containing 0.100 g of vitamin C dissolved in 10.0 mL of...
-
Below are two potential methods for preparing the same ether, but only one of them is successful. Identify the successful aproach and explain your choice. NaOMe ONa CH3I
-
Using acetylene as your only source of carbon atoms, design a synthesis of trans-5- decene:
-
On the maturity date of a $9,000, 9-month, 8% note, the borrower sends a check that includes the principal and all the interest due on the note. What is the amount of the borrowers check?
-
Ch . 5 - Quick Study 4 A company reports the following beginning inventory and two purchases for the month of January. On January 2 6 , the company sells 3 6 0 units. Ending inventory at January 3 1...
-
A deferred tax liability is created when Select one: a. the book basis of assets is greater than the tax basis of assets. b. the book basis of liabilities is greater than the tax basis of...

Study smarter with the SolutionInn App


