(a) In Exercise 12.21, if K 1 = 2.1 10 10 and K 2 = 2.3...
Question:
(a) In Exercise 12.21, if K1 = 2.1 × 1010 and K2 = 2.3 × 106, what is the value of K3?
(b) What is the equilibrium constant, K3′ for the reverse reaction?
Data from exercise 12.21
For the equilibria given below, determine each of the following:
Equilibrium expressions for K1 and K2
The equation for the reaction that is the sum of the two equations (c) the equilibrium expression, K3, for the sum of the two equations
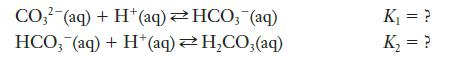
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





