Consider a sample of an ideal gas with n and T held constant. Which of the graphs
Question:
Consider a sample of an ideal gas with n and T held constant. Which of the graphs below represents the proper relationship between P and V? How would the graph differ for a sample with a larger number of moles?
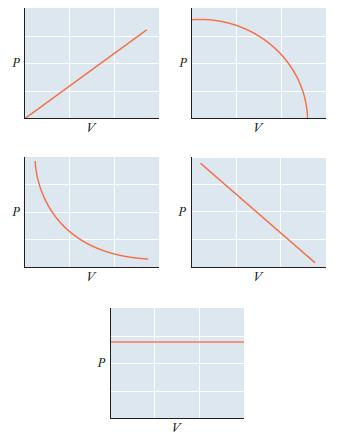
Transcribed Image Text:
P P V V P P P V V V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The graph on the left represents the proper relationship between P and V for a sample of an ideal ga...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
(1 point) Write the system of equations as a matrix equation, that is, rewrite it in the form Input your answer below: a11 a12 a13 = a21 = a22= = a23 = a31 b3 = a32 = a33 = b = = b2 = || || - -2x +...
-
Consider 2.00 moles of an ideal gas that is taken from state A (PA = 2.00 atm, VA = 10.0 L) to state B (PB = 1.00 atm, VB = 30.0 L) by two different pathways. These pathways are summarized in the...
-
In 1858, J. Waterston found a clever way to estimate molecular sizes from macro- scopic properties of a liquid, by comparing its surface tension and heat of vaporiza- tion. The surface tension of...
-
A firm has total debt of $6,000,000 and stockholder's equity is $4,000,000. The firm wants to calculate equity-to- total asset ratio in order to make decision about further raise of capital. What is...
-
Palmer Corporation operates on a calendar-year basis. It begins the annual budgeting process in late August when the president establishes targets for the total dollar sales and net income before...
-
A course in economics was taught to two groups of students, one in a classroom situation and the other online. There were 24 students in each group. The students were first paired according to...
-
4. Suppose the stock price is $35 and the continuously compounded interest rate is 5%. a. What is the 6-month forward price, assuming dividends are zero? b. If the 6-month forward price is $35.50,...
-
From its first day of operations to December 31, 2017, Campbell Corporation provided for uncollectible accounts receivable under the allowance method: entries for bad debt expense were made monthly...
-
BOOK Schedule of Cath Payments for a Service Company Horizon Financial Inc. was organicred on February 26. Projected selling and administrative expenses for each of the first three months of...
-
Consider a sample of N 2 gas under conditions in which it obeys the ideal gas law exactly. Which of these statements is true? (a) A sample of Ne(g) under the same conditions must obey the ideal gas...
-
Why do heavier gases move more slowly than light gases at the same temperature?
-
What do you find most compelling about Paul Meyers life story?
-
Account is a domestic growth portfolio. The current holdings are primarily US exchange-traded stocks and bonds. To remain in compliance, the total portfolio may only invest up to a maximum of 5% in...
-
The President of the United States needs your help. He has asked you to investigate and find answers to several important questions. His questions are included in the Letter from the President below....
-
Cheng Co. reports the following information for the coming year. Labor rate, including fringe benefits Annual labor hours Annual materials purchases Annual overhead costs: Materials purchasing,...
-
Compared to most objects, sound waves travel very fast. It is fast enough that measuring the speed of sound is a technical challenge. One method you could use would be to time an echo. For example,...
-
Sharif and Judith are married and purchased a vacation home together in Maine for $ 2 5 0 , 0 0 0 . Sharif died suddenly six months later and at that time the fair market value of the vacation home...
-
Midtown Holding Company operates numerous businesses, including motel, auto rental, and real estate companies. Year 20X6 was interesting for Midtown, which reported the following on its income...
-
Draw a Feynman diagram for the reaction n + v p + .
-
An ideal gas sample containing 1.75 moles for which C V ,m = 5/2R undergoes the following reversible cyclical process from an initial state characterized by T = 275 K and P = 1.00 bar: a. It is...
-
For protein denaturation, the excess entropy of denaturation is defined as is the transition excess heat capacity. The way in which δC trs P can be extracted from differential scanning...
-
The standard entropy of Pb(s) at 298.15 K is 64.80 J K -1 mol - 1 . Assume that the heat capacity of Pb(s) is given by The melting point is 327.4C and the heat of fusion under these conditions is...
-
A firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8....
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...

Study smarter with the SolutionInn App


