The standard entropy of Pb(s) at 298.15 K is 64.80 J K -1 mol - 1 .
Question:
The standard entropy of Pb(s) at 298.15 K is 64.80 J K-1mol-1. Assume that the heat capacity of Pb(s) is given by
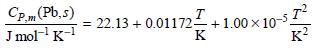
The melting point is 327.4°C and the heat of fusion under these conditions is 4770. J mol-1. Assume that the heat capacity of Pb(l) is given by
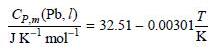
a. Calculate the standard entropy of Pb(l) at 725°C.
b. Calculate ΔH for the transformation Pb(s, 25.0°C) †’ Pb(l, 725°C).
Transcribed Image Text:
Cp (Pb,s) J mol- K- T 22.13 + 0.01172+1.00 x 10-3 K !! K?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
a b S Pb 199815 K SPb s 29815 K 99815 60055 S 29815 6...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data at 298.15 K. Assume that the heat capacities are independent of temperature. You are also given that...
-
Calculate the change of entropy, So, for the reaction given in Example 18.2a. The standard entropy of glucose, C6H12O6(s), is 212 J/(molK). See Table 18.1 for other values.
-
The dissociation vapour pressure ofNH4Cl at 427C is 608 kPa but at 459C it has risen to 1115 kPa. Calculate (a) The equilibrium constant, (b) The standard reaction Gibbs energy, (c) The standard...
-
Describe what is meant by the term balanced in the term balanced scorecard method.
-
Compare the results of the three (3) methods by quality of information for decision making. Using what you have learned about the three (3) methods, identify the best project by the criteria of long...
-
Some of the following passages contain explanations, some contain arguments, and some may be interpreted as either an argument or an explanation. What is your judgment about the chief function of...
-
What are the four Is of services?
-
The local residents of Greene County, a small, rural, mostly minority community, have recently learned that a major oil company is putting a refinery in the county. The residents ask for an...
-
EXERCISES AND PROBLEMS : FLEXIBLE BUDGET 1. The president of the company, Gregory Peters, has come to you for help. Use the following data to prepare a flexible budget for possible sales/production...
-
Write a program that will take as input two Web page URLs and find a path of links from one to the other. What is an appropriate search strategy is bidirectional search a good idea? Could a search...
-
For protein denaturation, the excess entropy of denaturation is defined as is the transition excess heat capacity. The way in which δC trs P can be extracted from differential scanning...
-
Under what conditions is S < 0 for a spontaneous process?
-
Add a % (remainder) operator to the expression calculator of Section 15.6.3. package collections; import java.util.Scanner; import java.util.Stack; public class ExpressionCalculator { public static...
-
Write a function that takes as input a non-negative integer in the range 0 to 99 and returns the English word(s) for the number as a string. Multiple words should be separated by a space. If the...
-
The Event Manager sighed as the festival approached and she had only five crafts vendors who had committed to taking part in the marketplace. She and her assistant were frantic. They had been...
-
the systematic recording, analysis, and interpretation of costs incurred by a business. Its significance extends beyond mere financial tracking; it plays a pivotal role in aiding management...
-
1.What is your process for ensuring that all your work is correct? 2.What do you mean by Batch Costing ? 3.Explain the accounting procedure for Batch Costing 4.State the applicability of Job Costing...
-
The increasing occurrence of freak weather incidents will have both local and global effects. Even in cases where production has been re-localized, freak weather can still greatly impact local...
-
In Exercises 3340, use the Intermediate Value Theorem to show that each polynomial has a real zero between the given integers f(x) = x 5 - x 3 - 1; between 1 and 2
-
How has the globalization of firms affected the diversity of their employees? Why has increased diversity put an additional burden on accounting systems?
-
Nickel is face-centred cubic with a unit cell of side 352 pm. What is the number of atoms per square centimeter exposed on a surface formed by (a) (100), (b) (110), (c) (11l) planes? Calculate the...
-
The data for the adsorption of ammonia on barium fluoride are reported below. Confirm that they fit a BET isotherm and find values of C and Vman' (a) 0= 0C, p* =429.6 kPa: p/kPa Vicm 106.4 16.5 14.0...
-
The adsorption of solutes on solids from liquids often follows a Freundlich isotherm. Check the applicability of this isotherm to the following data for the adsorption of acetic acid on charcoal at...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...

Study smarter with the SolutionInn App


