Considering the trends in electronegativity shown in Figure 7.7, explain why alloys that form between two or
Question:
Considering the trends in electronegativity shown in Figure 7.7, explain why alloys that form between two or more transition metals are not ionic substances.
Figure 7.7,
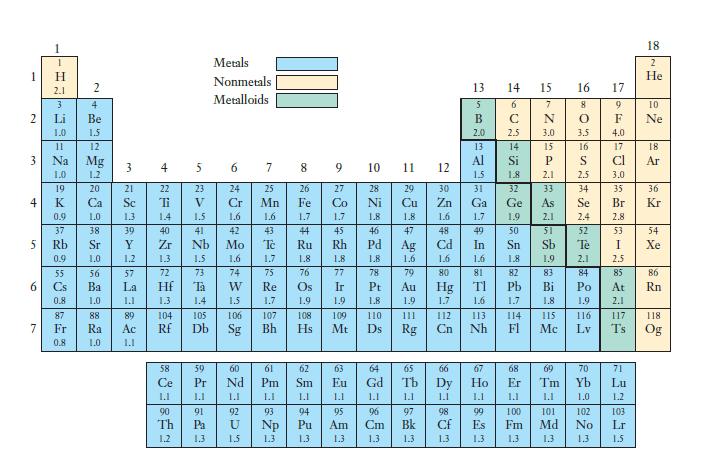
Transcribed Image Text:
1 H 2.1 3 Li Be 1.0 1.5 N 3 5 6 11 Na 1.0 19 K 0.9 37 Rb 0.9 55 Cs 0.8 87 Fr 0.8 2 12 Mg 1.2 20 56 Ba 1.0 88 21 SARIA 57 La 1.1 89 23 + N 1.3 77 58 Ce 1.1 90 Th 1.2 in 23 V 1.5 41 Nb 1.5 73 Ta 1.4 105 Db 50 Pr 1.1 91 Pa 1.3 Metals Nonmetals Metalloids 6 24 1.6 74 CASES 7 110 25 Mn 1.6 1.7 75 9 27 77 78 Ir Pr 1.9 Os 1.9 108 109 Hs Mt 28 29 1.1 95 Am 1.3 11 5 97 13 5 B 20 98 Cf 1.3 13 Al 15 31 Ga 1.7 1.6 81 TI 1.6 65 66 67 Tb Dv Ho 1.1 1.1 113 Nh 14 15 6 C 2.5 14 Si 1.8 32 82 Ph 1.7 68 Er 1.1 99 100 Es Fm 1.3 1.3 Its 15 21 33 83 Bi 1.8 115 Mc 16 17 8 0 3.5 16 S 2.5 34 Tè 2.1 84 Po 1.9 116 Lv SE 4.0 17 Cl 3.0 35 Br 2.8 53 I 2.5 85 At 2.1 71 Lu 1.2 103 Lr 1.5 18 86 Rn 118. Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Alloys The combination of two or more metals are called alloys For example brass is an alloy which i...View the full answer

Answered By

Ehsan Mahmood
I’ve earned Masters Degree in Business Studies and specialized in Accounts & Finance. Couple with this, I have earned BS Sociology from renowned institute of Pakistan. Moreover, I have humongous teaching experience at Graduate and Post-graduate level to Business and humanities students along with more than 7 years of teaching experience to my foreign students Online. I’m also professional writer and write for numerous academic journals pertaining to educational institutes periodically.
4.90+
248+ Reviews
287+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
a) Analysts attempt to value ordinary shares, in order to determine if market values are correct. Identify and describe the more common methods of valuation. Include a detailed explanation of the...
-
Briefly discuss the international capital markets. Citrus Products Inc. is a medium-sized producer of citrus juice drinks with groves in Indian River County, Florida. Until now, the company has...
-
Several factors are involved in the creation of a confidence interval. Among them are the sample size, the level of confidence, and the margin of error. Which statements are true? a) For a given...
-
How does the timing of hedges of (a) foreign currency denominated assets and liabilities, (b) foreign currency firm commitments, and (c) forecasted foreign currency transactions differ? LO9
-
What is the major task of promotion? Do firms ever use promotion to accomplish this task and fail? If so, give several examples.
-
Green Thumb Gardening is a small gardening service that uses activity-based costing to estimate costs for pricing and other purposes. The proprietor of the company believes that costs are driven...
-
Suppose that you wanted to make a diatomic molecule using two different halogen atoms. What combination of halogens would be the least polar? What combination would be the most polar?
-
In each group of three bonds, which bond is likely to be the most polar? Which will be the least polar? (a) CH, OH, SH, (b) CCl, ClCl, HCl, (c) FF, OF, CF, (d) NH, NO, NCl
-
Reflect on the concept of branding. Why do you think this would be important for your company?
-
Civil What are the challenges of using intermediate structures in the analysis of Pauli structures?
-
Civil What are the advantages of intermediate structures in the analysis of Laguerre structures?
-
Civil What are the disadvantages of using intermediate structures in the analysis of geothermal energy environmental product promotion?
-
Civil How can intermediate structures be used in the analysis of geothermal energy environmental product pulmonary toxins?
-
Civil What challenges arise when using intermediate structures in the analysis of structural robustness?
-
Review Example 14.1 and develop open and closed-loop control system equations for the force. Let the coefficient of friction be (?
-
Determine which of the following limits exist. Compute the limits that exist. lim x-0 1- + 3x X
-
Predict whether LiH + 2 and NH 2 should be linear or bent based on the Walsh correlation diagram in Figure 24.11. Explain your answers. Figure 24.11 1b, + 2a, 1b2 1a1 tog 100 120 140 160 180 Bond...
-
Use the framework described in Section 24.3 to construct normalized hybrid bonding orbitals on the central oxygen in O 3 that are derived from 2s and 2p atomic orbitals. The bond angle in ozone is...
-
Are the localized bonding orbitals in Equation (24.13) defined by And orthogonal? Answer this question by evaluating the integral « (ϲ) * Ï ²² dÏ. GC4. + C2Be2s...
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


