Consult a table of standard reduction potentials and determine which of the following reactions are spontaneous under
Question:
Consult a table of standard reduction potentials and determine which of the following reactions are spontaneous under standard electrochemical conditions.
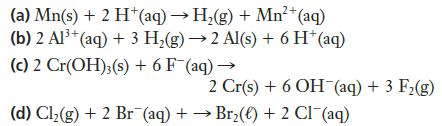
Transcribed Image Text:
2+ (a) Mn(s) + 2 H+ (aq) → H₂(g) + Mn²+ (aq) (b) 2 Al³+ (aq) + 3 H₂(g) → 2 Al(s) + 6 H* (aq) (c) 2 Cr(OH)3(s) + 6 F (aq) → 2 Cr(s) + 6 OH(aq) + 3 F₂(g) (d) Cl₂(g) + 2 Br (aq) + →Br₂() + 2 Cl¯(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a Spontaneou...View the full answer

Answered By

Peter Evans
I'm a Master's in Computer Application with 10 years of experience in the Information Technology Industry and 5 years of experience as a home tutor. As a tutor, I have extensive hands-on experience in providing individualized instruction to students in a variety of subject areas. I am highly proficient in breaking down complex topics into smaller, more manageable pieces, and I have a strong ability to explain concepts in a clear and concise way. I am experienced in creating engaging learning activities and finding innovative ways to keep students engaged and motivated. I also have experience in developing individualized learning plans to meet the specific needs of each student. Additionally, I am an expert in providing feedback and guidance to help students reach their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Suppose that you work for a company that designs the drive mechanisms for large ships. The materials in this mechanism will obviously come into contact with environments that enhance corrosion. To...
-
1. _____ is the process, procedures, and steps used to make sure digital certificates are up to date and stored securely. A. CSR B. CRL C. Key management D. CN 2. _____ is a type of...
-
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following reactions at 298 K. Data given in Appendix M (a) 3 Cu(s) + 2NO3(aq) + 8 H+ (aq) 3 Cu+ (aq) + 2 NO(g) +...
-
In the dynamic and expanding urban environment of "Slothsberg", a new highway ("Snailpace Highway") is to be constructed over the existing "Dillydally Highway". The following conditions apply to the...
-
Name eight or more ways to attract attention in opening a sales message.
-
Mukul is a teacher at Rockland School and he runs a tennis shop called Racket's Rackets. He and his wife Nikki have combined bank interest of $1,011. Nikki made $850 in tips last year. If they get a...
-
18-2. What is involved in sales management?
-
What does it mean to isolate the technical core of a business?
-
please show how to work out each question. Rick's is a popular restaurant for fine dining. The owner and chef, Rick Goetz, is pleased with his success and is now considering expanding his existing...
-
Use the potential of the galvanic cell, Co(s) Co 2+ 'IIPb 2+ IPb(s), to determine Gf(Pb 2+ ), given that Gf(Co 2+ ) = 54.4 kJ mol 1 .
-
Which of the following reactions is (are) spontaneous at standard conditions? 3+ 2+ (a) Zn(s) + 2 Fe+ (aq) Zn+ (aq) + 2 Fe+ (aq) (b) Cu(s) + 2 H+ (aq) Cu+ (aq) + H(g) (c) 2 Br (aq) + I(s) Br() +2...
-
Fergusons law enforcement practices are shaped by the Citys focus on revenue rather than by public safety needs. This emphasis on revenue has compromised the institutional character of Fergusons...
-
1. What is a forward contract? 2. Why do you need fx swap ? 3. If the rate is usd to cad, then you multiple by the rate. If converting USD to CAD. If the rate is CAD to USD, then you have to divide...
-
1. Watch the video on Taylorism. Why do you think Frederick Taylor's ideas were important at that particular point in history, the early 1900s? Explain. 2. How were the Hawthorne studies a major...
-
what extent do elite networks shape policy and governance, and how transparent are these networks to public scrutiny ? Explain
-
What strategies do you employ to mediate conflicts within a team to ensure that disagreements are resolved constructively and synergistically?
-
What goal(s) do you think the communication was intended to achieve? What type of promotional communication is it and why? What do you believe to be the advertising theme or central idea of the...
-
How does Joanne attempt to meet the, sometimes conflicting, requirements of customers and the workshop as she manages the planning and control system?
-
In the current year, the City of Omaha donates land worth $500,000 to Ace Corporation to induce it to locate in Omaha and create an estimated 2,000 jobs for its citizens. a. How much income, if any,...
-
The 60-mm-diameter shaft is made of 6061-T6 aluminum having an allowable shear stress of Ï allow = 80 MPa. Determine the maximum allowable torque T. Also, find the corresponding angle of twist...
-
The A-36 steel shaft has a diameter of 50 mm and is subjected to the distributed and concentrated loadings shown. Determine the absolute maximum shear stress in the shaft and plot a graph of the...
-
The splined ends and gears attached to the A992 steel shaft are subjected to the torques shown. Determine the angle of twist of end B with respect to end A. The shaft has a diameter of 40 mm. 400 N-m...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


