Which of the following reactions is (are) spontaneous at standard conditions? 3+ 2+ (a) Zn(s) + 2
Question:
Which of the following reactions is (are) spontaneous at standard conditions?
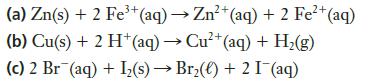
Transcribed Image Text:
3+ 2+ (a) Zn(s) + 2 Fe³+ (aq) → Zn²+ (aq) + 2 Fe²+ (aq) (b) Cu(s) + 2 H+ (aq) → Cu²+ (aq) + H₂(g) (c) 2 Br (aq) + I₂(s)→ Br₂() +2 I¯(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The reaction a Zns 2 Fe3aq Znaq 2 Feaq is not spontaneous at standard conditions ...View the full answer

Answered By

Gauri Hendre
I worked as EI educator for Eduphy India YT channel. I gave online tutorials to the students who were living in the villages and wanted to study much more and were preparing for NEET, TET. I gave tutions for topics in Biotechnology. I am currently working as a tutor on course hero for the biochemistry, microbiology, biology, cell biology, genetics subjects. I worked as a project intern in BAIF where did analysis on diseases mainly genetic disorders in the bovine. I worked as a trainee in serum institute of India and Vasantdada sugar institute. I am working as a writer on Quora partner program from 2019. I writing on the topics on social health issues including current COVID-19 pandemic, different concepts in science discipline. I learned foreign languages such as german and french upto A1 level. I attended different conferences in the science discipline and did trainings in cognitive skills and personality development skills from Lila Poonawalla foundation. I have been the member of Lila poonawalla foundation since 2017. Even I acquired the skills like Excel spreadsheet, MS Office, MS Powerpoint and Data entry.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Which of the following reactions are spontaneous in the direction written? See Table 18.2 for data. a. C(graphite) + 2H2(g) CH4(g) b. 2H2(g) + O2(g) 2H2O(l ) c. 4HCN(g) + 5O2(g) 2H2O(l) + 4CO2(g)...
-
A disproportionation reaction involves a substance that acts as both an oxidizing agent and a reducing agent, producing higher and lower oxidation states of the same element in the products. Which of...
-
From the values of H and S, predict which of the following reactions would be spontaneous at 25C: Reaction A: H 5 10.5 kJ/mol, DS 5 30 J/K ? mol; reaction B: H 5 1.8 kJ/mol, S 5 2113 J/K ? mol. If...
-
Use the ideas of Richardson's extrapolation and Romberg's method to evaluate the first derivative of f = ex at x = 0. To construct the first column of the Romberg's table, use the formula (exth -...
-
What could be included in an effective opening and closing of a claim or complaint letter?
-
Complete a 1040A form for Kevin Hooper. Kevin is a butcher. He is single with two children, which he can claim as dependents. He also takes care of his dad, who lives with his family. Use the...
-
18-3. What is the principal difference between an order taker and an order getter?
-
Listed below are brain volumes (cm3) of unrelated subjects used in a study. Use a 0.01 significance level to test the claim that the population of brain volumes has a mean equal to 1100.0 cm3. 963...
-
Vulcan Flyovers offers scenic overflights of Mount St. Helens, the volcano in Washington State that explosively erupted in 1982. Data concerning the company's operations in July appear below: Vulcan...
-
Consult a table of standard reduction potentials and determine which of the following reactions are spontaneous under standard electrochemical conditions. 2+ (a) Mn(s) + 2 H+ (aq) H(g) + Mn+ (aq)...
-
Suppose that you cannot find a table of standard reduction potentials. You remember that the standard reduction potential of Cu 2+ + 2 e Cu(s) is 0.337 V. Given that Gf(Cu 2+ ) = 65.49 kJ mol 1 and...
-
For household electrical outlets in the United States, the root-mean-square value of the source emf is \(120 \mathrm{~V}\). (a) For a hair dryer rated at \(1875 \mathrm{~W}\), what are the...
-
What Do You Know About Amazon Associate Program? What Would You Do To Increase Your Earnings With Amazon Associate Program? Is Affiliate Marketing And Referral Marketing One And The Same? What is...
-
As a leader, what do you think are important elements of a leadership team made up of those senior people that you will surround yourself with? Do you have (or have you had) a mentor? If so, how have...
-
What role do interorganizational relationships and alliances play in achieving strategic goals, and how do organizations manage these relationships to ensure mutual benefit and minimize risks ?
-
How do expatriate managers normally rotate into the operations of a foreign country? How long do they typically stay in the country? What are the disadvantages? How did Shane Tedjarati rotate into...
-
If you are not Asian, do you know someone well who is Asian? In what capacity do you know them (e.g., personal friend, manager, classmate, neighbor, etc.)? Do you know their ethnic origin (e.g.,...
-
What sequencing rules do you think the tower controllers use?
-
Access the Federation of Tax Administrators Internet site at www. taxadmin.org/state-tax-forms and indicate the titles of the following state tax forms and publications: a. Minnesota Form M-100 b....
-
The solid shaft of radius c is subjected to a torque T at its ends. Show that the maximum shear strain in the shaft is γ max = T c /JG. What is the shear strain on an element located at...
-
The propellers of a ship are connected to an A-36 steel shaft that is 60 m long and has an outer diameter of 340 mm and inner diameter of 260 mm. If the power output is 4.5 MW when the shaft rotates...
-
A motor delivers 500 hp to the shaft, which is tubular and has an outer diameter of 2 in. If it is rotating at 200 rad/s, determine its largest inner diameter to the nearest 1/8 in. if the allowable...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


