Determine the shape of the following molecules using VSEPR theory. (a) SF 4 , (b) BrF 5
Question:
Determine the shape of the following molecules using VSEPR theory.
(a) SF4,
(b) BrF5
Strategy As always, we start by drawing the Lewis structures. Then count the number of electron pairs around the central atom and determine the spatial arrangement of electron pairs, consulting Table 7.3 as necessary. Place the lone pairs in positions where the electron repulsions are minimized and describe the resulting geometric arrangement of the atoms.
Table 7.3
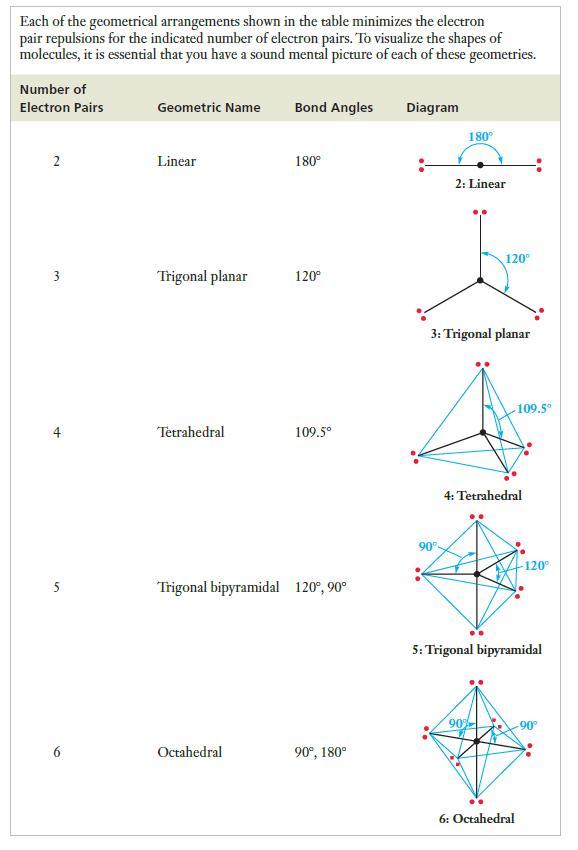
Transcribed Image Text:
Each of the geometrical arrangements shown in the table minimizes the electron pair repulsions for the indicated number of electron pairs. To visualize the shapes of molecules, it is essential that you have a sound mental picture of each of these geometries. Number of Electron Pairs 2 m t 5 6 Geometric Name Linear Trigonal planar Tetrahedral Bond Angles Octahedral 180° 120° 109.5° Trigonal bipyramidal 120°, 90° 90⁰, 180° Diagram 180° 90° 2: Linear 3: Trigonal planar 120⁰ 4: Tetrahedral 90% 109.5⁰ 5: Trigonal bipyramidal 6: Octahedral 120° -90°
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a The Lewis structure of SF 4 is as shown There are five pairs of electrons around sulfur so the shape with minimum interaction is a trigonal bipyrami...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Use VSEPR theory to determine the shape of the NOF molecule. Strategy Once again, we start by drawing the Lewis structure. Then count the number of regions of electron density around the central...
-
VSEPR (valence state electron pair repulsion) theory was formulated to anticipate the local geometry about an atom in a molecule (see discussion in Section 25.1). All that is required is the number...
-
For the instances mentioned below, identify the applicable laws/regulators. (Indicate multiple regulators, where applicable) a) Amalgamation of a weak private bank with another foreign private bank...
-
For problems involving composite bodies composed of two or more materials, the elasticity solution requires both boundary conditions and interface conditions between each material system. The...
-
Can activities 5 and 6 of Figure be eliminated? What risks does a project manager incur if these activities areeliminated? CONTRACTOR PROGRAM OFFICE REQUEST FOR HEDULES REVEL ROLIGH VERFY THAT ALL...
-
Why is the case of Haig v. Bamford considered important in the recent development of tort law?
-
Profit in marginal costing is more when production is more than sales.
-
Two coils are close to each other. The first coil carries a time varying current given by I (t) = (5.00 A) e-0.025 0t sin (377t). At t = 0.800 s, the emf measured across the second coil is -3.20 V....
-
A small company purchased now for $150,000 will lose $ 1000 each year for the first three years. An additional $5,000 in the company in the third year will result in a profit of $25,000 each year...
-
Although much less common than silicon devices, germanium-based semiconductors can also be fabricated. Which kind of material (n- or p-type) would result if pure germanium were doped with (a)...
-
Poly(vinyl alcohol) is used in several biomaterials applications, including surgical sutures. It is also used in drops for dry eyes and some contact lens solutions. Draw the Lewis structure of vinyl...
-
The following creep data were taken on an aluminum alloy at 400°C (750°F) and a constant stress of 25 MPa (3660 psi). Plot the data as strain versus time, then determine the steady-state or...
-
Use Table 19-4 to calculate the building, contents, and total property insurance premiums for the policy (in $). Area Structural Rating Class Building Value 4 B $86,000 $ Building Premium Contents...
-
What are some reasons why leadership theory has evolved? Which theory of leadership is most applicable to today's organizations? Identify a leader that you admire and answer the following: What makes...
-
Identifying one major OSHA standard and one EPA law that are important to aviation and discussing how each has improved aviation safety
-
What is network optimization and what are some of the best practices that are used in the industry to optimize networks? Also, why is network documentation important and what are the security...
-
Demonstrate your understanding of data types by examining a public dataset and identifying the NOIR analytical data types of each of the data field (variables). This skill will be used frequently in...
-
Kenzi Kayaking, a manufacturer of kayaks, began operations this year. During this first year, the company produced 1,050 kayaks and sold 800 at a price of $1,050 each. At this first year-end the...
-
How much more interest will be earned if $5000 is invested for 6 years at 7% compounded continuously, instead of at 7% compounded quarterly?
-
A weak acid has a dissociation constant of K a = 2.50 10 2 . a. Calculate the degree of dissociation for a 0.093m solution of this acid using the DebyeHckel limiting law. b. Calculate the degree of...
-
Calculate the mean ionic activity of a 0.0350 m Na 3 PO 4 solution for which the mean activity coefficient is 0.685.
-
At 25C, the equilibrium constant for the dissociation of acetic acid, K a , is 1.75 10 5 . Using the DebyeHckel limiting law, calculate the degree of dissociation in 0.150 m and 1.50 m solutions...
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


