Figure 7.2 depicts the interactions of an ion with its first nearest neighbors, second nearest neighbors, and
Question:
Figure 7.2 depicts the interactions of an ion with its first nearest neighbors, second nearest neighbors, and third nearest neighbors in a lattice.
Figure 7.2
(a) Would the interactions with the fourth nearest neighbors be attractive or repulsive?
(b) Based on Coulomb’s law, how would the relative sizes of the terms compare if the potential energy were expressed as V = V1st + V2nd + V3rd + V4th?
Transcribed Image Text:
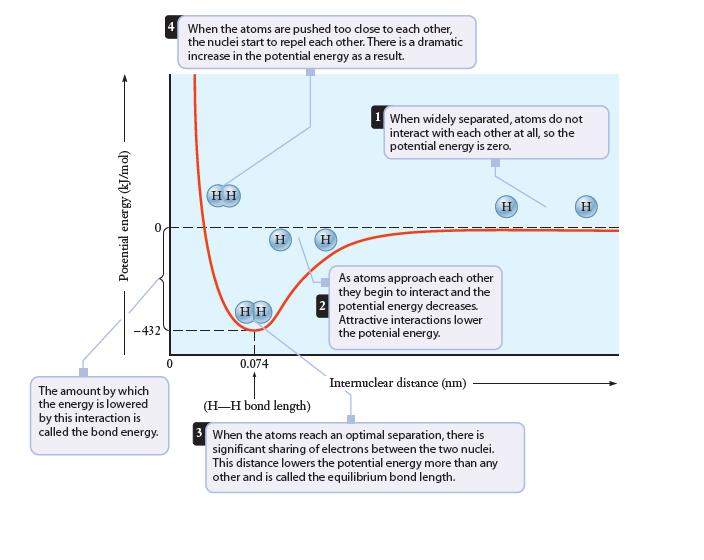
Potential energy (kJ/mol) -432 The amount by which the energy is lowered by this interaction is called the bond energy. 4 When the atoms are pushed too close to each other, the nuclei start to repel each other. There is a dramatic increase in the potential energy as a result. 0 HH HH 0.074 H H 1 When widely separated, atoms do not interact with each other at all, so the potential energy is zero. As atoms approach each other they begin to interact and the 2 potential energy decreases. Attractive interactions lower the potenial energy. Internuclear distance (nm) (H-H bond length) 3 When the atoms reach an optimal separation, there is significant sharing of electrons between the two nuclei. This distance lowers the potential energy more than any other and is called the equilibrium bond length. H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a Repulsive b According to Equation 72 the C...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Automobiles air bags are filled via the decomposition of sodium azide, according to the following equation: 2 NaN3 (s) 2 Na (s) +3 N2 (g) Calculate the work (in L atm) when 2.25 g of sodium azide...
-
What are holding companies? What are their advantages and disadvantages? Hagers Home Repair Company, a regional hardware chain that specializes in do-it-yourself materials and equipment rentals, is...
-
A student wants to investigate the effects of real vs. substitute eggs on his favorite brownie recipe. He enlists the help of 10 friends and asks them to rank each of 8 batches on a scale from 1 to...
-
On March 1, Pimlico Corporation (a U.S.-based company) expects to order merchandise from a supplier in Sweden in three months. On March 1, when the spot rate is $0.10 per Swedish krona, Pimlico...
-
The following profit payoff table was presented in Problem 1: The probabilities for the states of nature are P(s1) = 0.65, P(s2) = 0.15, and P(s3) = 0.20. a. What is the optimal decision strategy if...
-
On January 1, 2021, Darnell Window and Pane issued $18 million of 10-year, zero-coupon bonds for $5,795,518. (FV of $1, PV of $1, FVA of $1, PVA of $1, FVAD of $1 and PVAD of $1) (Use appropriate...
-
Arrange the following sets of anions in order of increasing ionic radii. (a) Cl , P 3 , S 2 , (b) S 2 , O 2 , Se 2 , (c) Br , N 3 , S 2 , (d) Br , Cl , I
-
Select the smaller member of each of the following pairs. (a) N and N 3 , (b) Ba and Ba 2+ , (c) Se and Se 2 , (d) Co 2+ and Co 3+
-
In addition to the suppositions of the preceding exercise, let g(x) > 0 for x (a, b), x c. If A > 0 and B = 0, prove that we must have If A limf(x)/g(x)-
-
2vx Voy Ax g 2vo cos 0 sin 0 g vo sin(20) g
-
PORTAGE COLLEGE Diversity Awareness Course Score | Home | Help | Exit Module 2 Post-Test Module 1 Module 2 Module 3 Module 2 Post-Test Betsy really likes working at Thompson Trucking. She likes how...
-
Suppose f(x) = 5x cos x. Find the equation of the tangent line to f(x) at the point (, -5). y = x+
-
First, for this case study, define the ethical dilemma facing "John". Second, isn't the collectability of an account ultimately based on opinion? If so , how does that play in the ethical dilemma...
-
Does the game have a dominant-strategy equilibrium? If so, what is it and why is it that? If not, why not?
-
Explain why sensors have become so essential in the development of automated manufacturing systems? Give some examples.
-
The Ferris wheel in the figure has a radius of 68 feet. The clearance between the wheel and the ground is 14 feet. The rectangular coordinate system shown has its origin on the ground directly below...
-
In the polyproline spectroscopic ruler experiment shown in Figure 25.19, the FRET pair employed is comprised of the fluorescent dyes Alexa Fluor 488 (excited-state lifetime of 4.1 ns) and Alexa Fluor...
-
For many years, a controversy raged concerning the structures of so-called electron-deficient molecules; that is, molecules with insufficient electrons to make normal two-atom, two-electron bonds....
-
One of the most powerful attractions of quantum chemical calculations over experiments is their ability to deal with any molecular system, stable or unstable, real or imaginary. Take as an example...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


