For the reaction of nitrogen, N 2 , and hydrogen, H 2 , to form ammonia, NH
Question:
For the reaction of nitrogen, N2, and hydrogen, H2, to form ammonia, NH3, a student is attempting to draw a particulate diagram, as shown below. Did the student draw a correct representation of the reaction? If not, what was the error the student made?
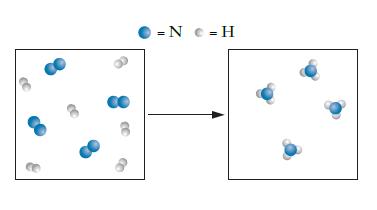
Transcribed Image Text:
= N c = H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
No the drawing is not correct ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
When your sympathetic nervous system is not activated and you don't have super- human powers, which hemisphere of your brain do you think you rely on most to process information? Under what...
-
Nitrogen (N2) and hydrogen (H2) react to form ammonia (NH3). Consider the mixture of N2 and H2 shown in the accompanying diagram. The blue spheres represent N, and the white ones represent H. Draw a...
-
For the reaction of nitrogen monoxide, NO, with chlorine, Cl2, 2NO(g) + Cl2(g) 2NOCl(g) the observed rate law is Rate = k[NO]2[Cl2] What is the reaction order with respect to nitrogen monoxide? with...
-
A money market portfolio has a market value of $20,000,000 and its value will change by $500 for a change in short-term yields of one basis point. The eurodollar futures contract has a tick size of...
-
The yields for Treasuries with differing maturities on a recent day were as shown in the table. a. Use the information to plot a yield curve for this date. b. If the expectations hypothesis is true,...
-
For 2019, the amount of the sales tax deduction is calculated by: a. Determining the actual sales tax paid during the year. b. Using the IRS sales tax deduction calculator. c. Using the sales...
-
What is a job analysis? Describe some of the questions which must be answered in preparing a job analysis. How does a job description differ from a job analysis? What are some of the considerations...
-
Atlantic Coast Railroad Company wishes to evaluate three capital investment proposals by using the net present value method. Relevant data related to the proposals are summarized as follows:...
-
QUESTION 1 Syafiqah is an expatriate working in the Kuala Lumpur Headquarters of Mega Infrastructure Corp. She was transferred to the current office from 10 June 2013 as a Regional Managing Director...
-
The picture shown depicts the species present at the start of a combustion reaction between methane, CH 4 , and oxygen, O 2 . (a) Draw the resulting state after this set of reactants has reacted as...
-
Carbon dioxide is just one of many greenhouse gases in the atmosphere. What property makes a gas a greenhouse gas?
-
The intensity I of light at a depth of x meters below the surface of a lake satisfies the differential equation dI/dx = (-1.4)I. (a) At what depth is the intensity half the intensity I0 at the...
-
NOT ASKING THE ACTUAL SHEAR STRESS. Please READ! Derive the shear stress distributed equation over the cross-section. Derive the equation and plot. 15 15 30 15 15 120 -90 20 0.5 m 72 kN 20 20 40 40...
-
You are the cost accountant of an engineering concern which has three departments - preparation, machining and assembly. The budgeted direct labour hours for the workshops are 8,000, 12,000 and...
-
What alternative to fostering fun and enjoyment at work do you think might have worked for Zappos?
-
Using the techniques of dimensional analysis, and assuming that experimentation shows the dimensionless number to be 1, derive the following equation: E v = Job card two The results of an ultrasonic...
-
Given the historical cost of product Carla Vista is $13, the selling price of product Carla Vista is $15, costs to sell product Carla Vista are $3, the replacement cost for product Carla Vista is...
-
How do econometric models differ from "naive" projection methods? Is it always advisable to use the former in forecasting?
-
In the current year, the City of Omaha donates land worth $500,000 to Ace Corporation to induce it to locate in Omaha and create an estimated 2,000 jobs for its citizens. a. How much income, if any,...
-
Propose a plausible mechanism for the following process, called iodolactonization: I2
-
When 3-bromocyclopentene is treated with HBr, the observed product is a racemic mixture of trans-1,2-dibromocyclopentane. None of the corresponding cis-dibromide is observed. Propose a mechanism that...
-
Provide a systematic name for each of the following compounds: (a) (b) (c) (d)
-
What would be the price of a 1000 5% 10 year bond if the market rate was 6%
-
Calgon Company has a cost of equity of 10.2 percent, a pretax cost of debt of 7 percent, and a tax rate of 21 percent. The company's capital structure consists of debt as 38 percent of the company's...
-
Farm Management True or False Please explain answer. Thank you. A. When you acquire machinery under a financial lease, only that part of the payment that is interest is a tax-deductible expense. B....

Study smarter with the SolutionInn App


