Question: For the system in the preceding problem, show the equilibrium condition in terms of the rates of the forward and reverse reactions. Data from Preceding
For the system in the preceding problem, show the equilibrium condition in terms of the rates of the forward and reverse reactions.
Data from Preceding Problem
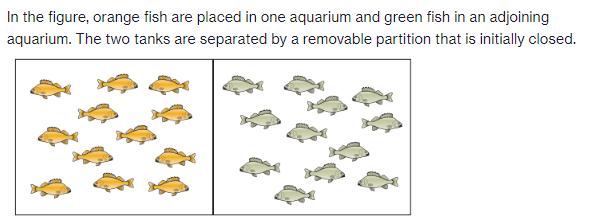
In the figure, orange fish are placed in one aquarium and green fish in an adjoining aquarium. The two tanks are separated by a removable partition that is initially closed.
Step by Step Solution
3.47 Rating (150 Votes )
There are 3 Steps involved in it
The equilibrium condition for the system in the preceding problem is that the rates of the forward a... View full answer

Get step-by-step solutions from verified subject matter experts


