Hydrazine, N 2 H 4 , has been proposed as the fuel in a fuel cell in
Question:
Hydrazine, N2H4, has been proposed as the fuel in a fuel cell in which oxygen is the oxidizing agent. The reactions are
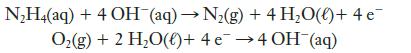
(a) Which reaction occurs at the anode and which at the cathode?
(b) What is the net cell reaction?
(c) If the cell is to produce 0.50 A of current for 50.0 h, what mass in grams of hydrazine must be present?
(d) What mass in grams of O2 must be available to react with the mass of N2H4 determined in part (c)?
Transcribed Image Text:
N₂H4(aq) + 4 OH¯(aq) → N₂(g) + 4H₂O(l) + 4e¯ O₂(g) + 2 H₂O()+ 4 e 4 OH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Which reaction occurs at the anode and which at the cathode The anode is the negative electrode an...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The large magnetic fields used in MRI can produce forces on electric currents within the human body. This effect has been proposed as a possible method for imaging biocurrents flowing in the body,...
-
A study is to be made using liquid ammonia as the fuel in a gas-turbine engine. Consider the compression and combustion processes of this engine. a. Air enters the compressor at 100 kPa, 25C, and is...
-
A study is to be made using liquid ammonia as the fuel in a gas-turbine engine. Consider the compression and combustion processes of this engine. a) Air enters the compressor at 100 kPa, 25C,...
-
If you could choose, which type of school would you want your imaginary child(ren) to attend?
-
Compare and contrast the methodologies used by Interbrand (www.interbrand.com) and BrandZ (www.brandz.com) to determine brand value. Explain why there is a discrepancy in the rankings from these two...
-
Researchers have characterized the niches of Darwin's finches by beak size (which correlates with diet) and the niches of salt marsh grasses by position in the intertidal zone. How would you...
-
Protective packaging. Goods moving in a distribution system must be contained, protected, and identified. In addition, goods are moved and stored in packages and must fit into the dimension of the...
-
The box plot shows the undergraduate in-state charge per credit hour at four-year public colleges. a. Estimate the median. b. Estimate the first and third quartiles. c. Determine the interquartile...
-
A deposit of 1 is made at the end of each year for 10 years into a bank account that pays interest at the end of each year at an annual effective rate of j. Each interest payment is reinvested into...
-
Suppose that, prior to other firms entering the market, the maker of a new smartphone (Way Cool, Inc.) earns $100 million per year. By reducing its price by 50 percent, Way Cool could discourage...
-
A current is passed through a solution of copper(II) sulfate long enough to deposit 14.5 g of copper. What volume of oxygen is also produced if the gas is measured at 24C and 0.958 atm of pressure?
-
Marginal distributions arent the whole story Here are the row and column totals for a two-way table with two rows and two columns: a b 50 c d 50 60 40 100 Find two different sets of counts a, b, c,...
-
Mijka Company was started on January 1, Year 1. During Year 1, the company experienced the following three accounting events: 1. earned cash revenues of $32,500 2. paid cash expenses of $14,500 3....
-
Q2. Find the equations of the tangent and normal to the curve x3 + y = 2 at (1, 1). Q3. Find if y dx y= :xsinx + (sinx)cosx [10] [10]
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
The formula weight (FW) of a gas can be determined using the following form of the ideal gas law FW = g R T / PV where g is the mass in grams, R is the gas constant, T is the temperature in Kelvin, P...
-
Consider a game in which a fair die is thrown. The player pays $5 to play and wins $2 for each dot that appears on the roll. Define X = number on which the die lands, and Y = player's net profit...
-
Who do you think should manage the Laz-skan Development Project?
-
Find i 0 (t) for t > 0 in the circuit in Fig. 16.72 . 2 + Vo 1 7.5e-2t u(t) V ( +) 4.5[1 u(t)]V 0.5v. 1H
-
The gear motor can develop 2 hp when it turns at 150 rev/min. If the allowable shear stress for the shaft is Ï allow = 8 ksi, determine the smallest diameter of the shaft to the nearest 1/8 in....
-
The gear motor can develop 1/4 hp when it turns at 600 rev/min. If the shaft has a diameter of 1/2 in., determine the maximum shear stress in the shaft.
-
The gear motor can develop 1/10 hp when it turns at 80 rev/min. If the allowable shear stress for the shaft is Ï allow = 4 ksi, determine the smallest diameter of the shaft to the nearest 1/8...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


