In Example Problem 12.2, we saw that hydroxide ions precipitate with calcium. Magnesium ions show similar behavior.
Question:
In Example Problem 12.2, we saw that hydroxide ions precipitate with calcium. Magnesium ions show similar behavior. The two pertinent equilibria are:
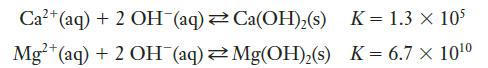
Which ion is more likely to precipitate hydroxide from a solution, assuming roughly equal concentrations of calcium and magnesium ions?
Strategy The likely extent of each reaction can be predicted by the size of the equilibrium constant. By comparing the two numbers, we can see which cation is more likely to precipitate the hydroxide.
Example Problem 12.2
Calcium hydroxide will precipitate from solution by the following equilibrium:
![]()
Write the equilibrium expression for this reaction.
Strategy Write the equilibrium expression as before but do not include a term for the concentration of the solid calcium hydroxide product.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





