Many reactions occur in the formation of photochemical smog, including the reaction of ozone with various volatile
Question:
Many reactions occur in the formation of photochemical smog, including the reaction of ozone with various volatile organic compounds, or VOCs. The following table shows the Arrhenius expressions for four such reactions.
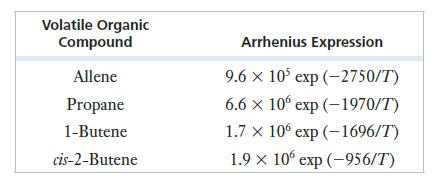
(a) Which reaction is likely to be the fastest at 310 K?
(b) Which reaction has the lowest activation energy?
(c) Which has the highest activation energy?
Transcribed Image Text:
Volatile Organic Compound Allene Propane 1-Butene cis-2-Butene Arrhenius Expression 9.6 x 105 exp(-2750/T) 6.6 x 10 exp (-1970/T) 1.7 x 10 exp (-1696/T) 1.9 × 10 exp (-956/T)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine which reaction is likely to be the fastest at 310 K we need to compare the Arrhenius expressions for each reaction The Arrhenius expressi...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
One of the chemical reactions that occurs in the formation of photochemical smog is O 3 + NO NO 2 + O 2 . Estimate r H for this reaction by using appropriate Lewis structures and data from Table...
-
Evaluate 3x - 1 / x - 1. lim
-
The company had the following shares of stock outstanding during the year. Data regarding dividend privileges and net income are also given. Common shares outstanding: 320,000 for the entire year...
-
Are ethics and corporate governance important topics in the study of accounting and the business environment? Why?
-
Is there something unique or distinctive about the product or service that separates it from substitutes and competitors?
-
Teresas manufacturing plant is destroyed by fire. The plant has an adjusted basis of $260,000, and Teresa receives insurance proceeds of $400,000 for the loss. Teresa reinvests $425,000 in a...
-
a. Raw materials of $36,000 were requisitioned to the factory. An analysis of the material requisition slips indicated that $6,800 was classified as indirect materials. b. Factory labor costs...
-
Can a reaction mechanism ever be proven, correct? Can it be proven incorrect?
-
The table below presents rate constants measured at three temperatures for the following reaction, which is involved in the production of nitrogen oxides in internal combustion engines. (Assume the...
-
A review of the ledger of Khan Company at December 31, 2017, produces the following data pertaining to the preparation of annual adjusting entries. 1. Prepaid Insurance ¬9,300. The company has...
-
Describe in your own words how you would expect the data points on a scatterplot to be distributed if the following features were present (i.e. for each part, explain how the feature would look on a...
-
imagine this experimental setup: One temperature probe is in embedded in a small block of frozen sugar water at -20. The frozen sugar water is in a small test tube The melting/freezing point of this...
-
Question 2: (40 points: 10 each) During September, Sweet Foods manufactures a single product. The Company's material purchases amounted to 9,000 pounds at a price of $9.80 per pound. Actual costs...
-
E12-23 (Algo) (Supplement 12B) Preparing a Statement of Cash Flows, Indirect Method: T-Account Approach [LO 12-S2] Golf Goods Incorporated is a regional and online golf equipment retailer. The...
-
A symmetric compound channel in over bank flow has a main channel with a bottom width of 30 m, side slopes of 1:1, and a flow depth of 3m. The floodplains on either side of the main channel are both...
-
The substance l-butyl-3-methylimidazolium (BMIM) hexafluorophosphate (margin) is a liquid at room temperature, even though it is a salt composed of positive and negative ions. BMIM and other ionic...
-
7 A 29-year-old, previously healthy man suddenly collapses at a party where legal and illicit drugs are being used. Enroute to the hospital, he requires resuscitation with defibrillation to establish...
-
The KermackMcKendrick model was developed to explain the rapid rise and fall in the number of infected people during epidemics. This model involves the interaction of susceptible (S), infected (I),...
-
Sunburn is caused primarily by sunlight in what is known as the UVB band, or the wavelength range from 290 to 320 nm. The minimum dose of radiation needed to create a sunburn (erythema) is known as...
-
A likely mechanism for the photolysis of acetaldehyde is Derive the rate law expression for the formation of CO(g) based on this mechanism. CH CHO (s) + iv CH;-(g) + C-(g) CH,- (9) + ,e) H,(8) +...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


