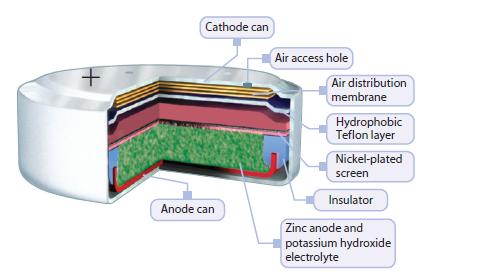
The electrolyte solution in a zinc-air battery (Figure 13.16) is aqueous KOH. For a zinc-air battery to
Question:
The electrolyte solution in a zinc-air battery (Figure 13.16) is aqueous KOH. For a zinc-air battery to obtain O2 from the air, there are tiny openings in the battery. However, these openings also allow water vapor to escape and enter the battery as the humidity changes. The optimum relative humidity for efficient zinc-air battery function, at which the KOH electrolyte is in equilibrium with water in the surrounding air, is 60% at 25°C. On the molecular level, describe what will happen to a zinc-air battery if used consistently in
(a) An arid environment and
(b) An extremely humid environment.
Figure 13.16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





