The following oxidationreduction reactions are used in electrochemical cells. Write them using cell notation. a. b. c.
Question:
The following oxidation–reduction reactions are used in electrochemical cells. Write them using cell notation.
a.
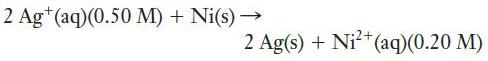
b.
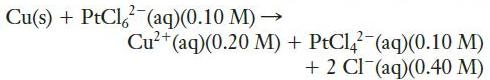
c.
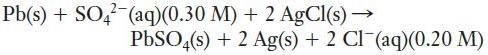
d. In a galvanic cell, one half-cell contains 0.010 M HCl and a platinum electrode, over which H2 is bubbled at a pressure of 1.0 atm. The other half-cell is composed of a zinc electrode in a 0.125 M solution of Zn(NO3)2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





