The following series of pictures represents the progress of a reaction in which A 2 molecules dissociate
Question:
The following series of pictures represents the progress of a reaction in which A2 molecules dissociate into atoms: A2 → 2 A. Each picture represents a snapshot of the reaction mixture at the indicated time.
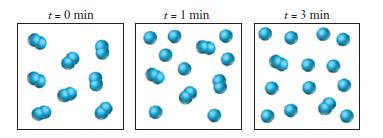
Are these figures consistent with a first-order rate law?
Explain.
Transcribed Image Text:
t = 0 min t = 1 min t = 3 min
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
If we use the number of A 2 molecules in the picture to repres...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
In 1987, U.S. controlled companies earned an average 2.09% return on assets, nearly four times their foreign controlled counterparts. A number of American politicians have used these figures to argue...
-
Assessing the impact of an incentive plan overview ladbrecks is a major department store with fifty retail outlets. The company's stores compete with outlets run by companies such as nordstrom,...
-
The hydroxyl radical, OH, is formed at low altitudes via the reaction of excited oxygen atoms with water: (a) Write the Lewis structure for the hydroxyl radical. Once produced, the hydroxyl radical...
-
Describe what is meant by the supply chain. (1marks) 2. Using examples from the case study, analyse how Nestle's Creating Shared Value contributes to its corporate social responsibility activities....
-
For each of the following items, indicate whether the expense should be recognized using (1) direct matching, (2) systematic and rational allocation, or (3) immediate recognition. Provide support for...
-
For each of the following linear systems, obtain a solution by graphical methods, if possible. Explain the results from a geometrical standpoint. a. x1 + 2x2 = 3, x1 x2 = 0. b. x1 + 2x2 = 3, 2x1 +...
-
Do blondes raise more funds? During fundraising, does the physical appearance of the solicitor affect the level of capital raised? An economist at the University of Nevada Reno designed an experiment...
-
Hovington, CPA, knows that while audit objectives relating to inventories may be stated in terms of the assertions as presented in this chapter, they may also be subdivided and stated more...
-
The following balance sheet information was provided by O'Connor Company: Assets Year 2 Year 1 Cash $ 4,000 $ 2,000 Accounts receivable 15,000 12,000 Inventory $ 35,000 $ 38,000 Assuming that net...
-
The rate of photodecomposition of the herbicide picloram in aqueous systems was determined by exposure to sunlight for a number of days. One such experiment produced the following results. (Data from...
-
Substances that poison a catalyst pose a major concern for many engineering designs, including those for catalytic converters. One design option is to add materials that react with potential poisons...
-
Suggest reasons why the format and content of financial accounting reports tend to be more standardized than the format and content of accounting reports that firms prepare for their internal...
-
A new partner C is invited to join in the AB partnership. Currently, A's and B's capital are $540,000 and $100,000, respectively. According to their profit and loss sharing contract, partner A and B...
-
The two tanks shown are connect through a mercury manometer. What is the relation between ???? and ? water Az water Ah
-
1. After reading about the types of rights that prisoners have while incarcerated, which of these rights, if any, should be reduced or diminished? Why? 2. In the same way, what rights do you believe...
-
According to the Socratic view of morality summarized by Frankena, is a person brought up by immoral parents in a corrupt society capable of making correct moral judgements? Why or why not? Do you...
-
Loma Company manufactures basketball backboards. The following information pertains to the company's normal operations per month: Output units15,000 boards Machine-hours4,000 hours Direct...
-
Which of the following cycloalkanes has the greatest ring strain? (a) Cyclopropane (b) Cyclobutane (c) Cyclohexane (d) Cycloheptane
-
You deposit $10,000 in a savings account that earns 7.5% simple interest per year. What is the minimum number of years you must wait to double your balance? Suppose instead that you deposit the...
-
Consider a process that attempts to prepare tyrosine using a HellVolhardZelinski reaction: (a) Identify the necessary starting carboxylic acid. (b) When treated with Br 2 , the starting carboxylic...
-
Proton NMR spectroscopy provides evidence for the restricted rotation of a peptide bond. For example, N,Ndimethylformamide exhibits three signals in its proton NMR spectrum at room temperature. Two...
-
We saw in Section 25.6 that DCC can be used to form a peptide bond. We explored the mechanism, and we saw that DCC activates the COOH moiety so that it readily undergoes nucleophilic acyl...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


