The graph below shows G as a function of temperature for the synthesis of ammonia from nitrogen
Question:
The graph below shows ΔG° as a function of temperature for the synthesis of ammonia from nitrogen and hydrogen.
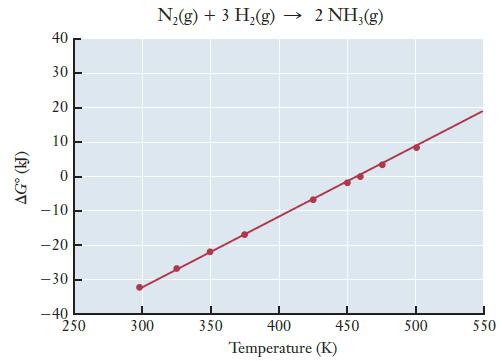
(a) Use the graph to estimate ΔS° for the ammonia synthesis reaction.
(b) Given that the standard free energy change of formation for ammonia (ΔGf°) is –16.50 kJ/mol, estimate ΔH° for the ammonia synthesis reaction.
Transcribed Image Text:
AGⓇ (kJ) 40 30 20 10 0 -10 -20 -30 -40 1 250 300 N₂(g) + 3 H₂(g) → 2 NH3(g) 350 450 400 Temperature (K) 500 550
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a 2...View the full answer

Answered By

Parvesh Kumar
I am an experienced Mathematics and Statistics tutor with 10 years of experience teaching students and working professionals. I love teaching students who are passionate to learn subjects or wants to understand any mathematics and statistics concept at graduation or master’s level. I have worked with thousands of students in my teaching career. I have helped students deal with difficult topics and subjects like Calculus, Algebra, Discrete Mathematics, Complex analysis, Graph theory, Hypothesis testing, Probability, Statistical Inference and more. After learning from me, students have found Mathematics and Statistics not dull but a fun subject. I can handle almost all curriculum of mathematics. I did B.Sc (mathematics), M.Sc (mathematics), M.Tech (IT) and am also Gate (CS) qualified. I have worked in various college and school and also provided online tutoring to American and Canadian students. I look forward to discussing with you and make learning a meaningful and purposeful
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Ammonia is produced directly from nitrogen and hydrogen by using the Haber process. The chemical reaction is N2 (g) + 3H2 (g) 2NH3 (g) (a) Use Table 8.4 to estimate the enthalpy change for the...
-
The catalytic reaction A B takes place within a fixed bed containing spherical porous catalyst X22. Figure P15-2B shows the overall rates of reaction at a point in the reactor as a function of...
-
Ammonia is produced by the Haber process, in which nitrogen and hydrogen are reacted directly using an iron mesh impregnated with oxides as a catalyst. For the reaction N2(g) + 3H2(g) 2NH3(g)...
-
A psychologist shows a list of eight activities to a subject in an experiment. How many ways can the subject pick a first, second, and third activity? a. Identify the total number of objects n and...
-
The Anson Manufacturing Company reviewed its year-end inventory and found the following items. Indicate which items should be included in the inventory balance at December 31, 2011. Give your reasons...
-
Give a brief description of DHCP operation.
-
What is Project Quality Management? AppendixLO1
-
Ehrlich Co. began business on January 2, 20Y8. Salaries were paid to employees on the last day of each month, and social security tax, Medicare tax, and federal income tax were withheld in the...
-
A(n) eight-year bond has a yield of 10% and a duration of 7.196 years. If the bond's yield increases by 30 basis points, what is the percentage change in the bond's price? (Input the value as a...
-
Suppose that you need to know the heat of formation of cyclohexane, C 6 H 12 , but the tables you have do not provide the value. You have a sample of the chemical. What could you do to determine the...
-
Using only the data given below, determine G for the following reaction: NO(g) + O(g) NO(g) (Remember that AG is a state function, just like AH.) 2 O3(g) 3 O(g) AG= -326 kJ O(g) 2 0(g) AG = 463.6...
-
What is digitizing a signal?
-
Listed below are the lead concentrations (in ug/g) measured in different Ayurveda medicines. Ayurveda is a traditional medical system commonly used in India. The lead concentrations listed here are...
-
The assignment states to use a movie and talk about 2 scenes where physics ideas are used. The rubric is shown and 6 big ideas that can be talked about are also attached. Background Information...
-
A series of computer and backup system failures caused the loss of most of the company records at Stotter, Incorporated. Information technology consultants for the company could recover only a few...
-
Future value of an annuity Using the values below, answer the questions that follow. (Click on the icon here in order to copy the contents of the data table below into a spreadsheet.) Deposit period...
-
Mercury, Incorporated, produces cell phones at its plant in Texas. In recent years, the company's market share has been eroded by stiff competition from overseas. Price and product quality are the...
-
In a ternary amplitude shift keying (ASK) communications system, there are three equally likely transmitted signals {s0, s1, s2}. These signals are distinguished by their amplitudes such that if...
-
A parking lot charges $3 for the first hour (or part of an hour) and $2 for each succeeding hour (or part), up to a daily maximum of $10. (a) Sketch a graph of the cost of parking at this lot as a...
-
Determine the number of permutations of size 3 that can be made from the set {1, 2, 3, 4, 5, 6}. Write down all of the permutations.
-
Determine the numerical values for the following: a. The number of configurations employing all objects in a six-object set. b. The number of configurations employing four objects from a six-object...
-
Radio station call letters consist of four letters (for example, KUOW). a. How many different station call letters are possible using the 26 letters in the English alphabet? b. Stations west of the...
-
ABC company makes turbo-encabulators, customized to satisfy each customers order. They split overhead into five pools, each with its own activity driver (direct labor for manufacturing, direct labor...
-
Variable manufacturing overhead becomes part of a unit's cost when variable costing is used.Group of answer choicesTrueFalse
-
Santa Fe Corporation has computed the following unit costs for the year just ended:Direct Material used $23Direct Labor $18Fixed selling and administrative cost $18Variable manufacturing overhead...

Study smarter with the SolutionInn App


