Using only the data given below, determine G for the following reaction: NO(g) + O(g) NO(g)
Question:
Using only the data given below, determine ΔG° for the following reaction:
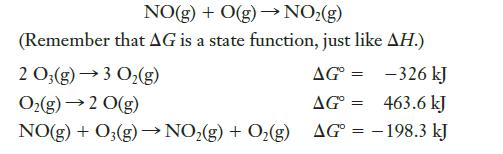
Transcribed Image Text:
NO(g) + O(g) → NO₂(g) (Remember that AG is a state function, just like AH.) 2 O3(g) →3 O₂(g) AG= -326 kJ O₂(g) → 2 0(g) AG = 463.6 kJ NO(g) + O₂(g) →→NO₂(g) + O₂(g) AG = -198.3 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To determine G for the reaction NOg Og NO2gwe can use the following Hesss Law cycl...View the full answer

Answered By

Aun Ali
I am an Associate Member of Cost and Management Accountants of Pakistan with vast experience in the field of accounting and finance, including more than 17 years of teaching experience at university level. I have been teaching at both undergraduate and post graduate levels. My area of specialization is cost and management accounting but I have taught various subjects related to accounting and finance.
5.00+
13+ Reviews
32+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Using the data given below that relate to the formation of Schottky defects in some oxide ceramic (having the chemical formula MO), determine the following: (a) The energy for defect formation (in...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Using only the data given in the text, what is the maximum number of telephones that the existing U.S. system can support without changing the numbering plan or adding additional equipment? Could...
-
1. A drying process is used to remove 3000 lbm/h of water (vapor) at steady-state from processed paper. The dryer first heats atmospheric air (state 1) at 80F, 1 atm and 60% relative humidity to 250F...
-
Judge Daniel H. Wells is currently deliberating over a suit filed by three stockholders against Transcontinental Corporation. The stockholders allege that Transcontinentals year-end balance sheet was...
-
What is Mosaic?
-
What is Project Human Resources Management? AppendixLO1
-
1. In preparing a statement of cash flows, the cost of acquiring a subsidiary is reported: a. As an operating activity under the direct method b. As an operating activity under the indirect method c....
-
Suppose a portfolio is given as follows: What is the expected return of the portfolio? 6.0% 6.5% 7.0% 6.3% none listed
-
The graph below shows G as a function of temperature for the synthesis of ammonia from nitrogen and hydrogen. (a) Use the graph to estimate S for the ammonia synthesis reaction. (b) Given that the...
-
The reaction shown below is involved in the refining of iron. (The table that follows provides all of the thermodynamic data you should need for this problem.) (a) Find H for the reaction. (b) S for...
-
Recall from Chapter 13 that Hincapie Co. (a specialty bike-accessory manufacturer) is expecting growth in sales of some products targeted to the low-price market. Hincapie is contemplating a...
-
Hey i need the following pages done ASAP.Please and thank you D2L Lesson 1: Electrostatics - Physics X L1Electrostatics X Course Hero +...
-
Bed & Bath, a retailing company, has two departmentsHardware and Linens. The companys most recent monthly contribution format income statement follows: Department Total Hardware Linens Sales $...
-
Use the formula g'(a) = lim. [(g(x)-g(a) )/ (x-a) ] x-->a to find g'(x) for g(x) = srqrt(x+7). Find the equation fo the tangrnt line to g(x) at x =-5
-
As shown in the figure below, an incompressible liquid flows steadily and vertically upward in a circular cross-sectional pipe. At section (1), the velocity profile over the cross-sectional area is...
-
writE a paper on teamwork 1. Share an experience with working on teamwork during your academic journey or on the job. 2. Share both positive and negative experiences. 3. What are your suggestions...
-
A binary communication system has transmitted signal X, the Bernoulli (1/2) random variable. At the receiver, we observe Y = VX + W, where V is a "fading factor" and W is additive noise. Note that V...
-
Prove the formula for (d/dx)(cos-1x) by the same method as for (d/dx)(sin-1x).
-
Four bases (A, C, T, and G) appear in DNA. Assume that the appearance of each base in a DNA sequence is random. a. What is the probability of observing the sequence AAGACATGCA? b. What is the...
-
The natural abundance of 13 C is roughly 1%, and the abundance of deuterium ( 2 H or D) is 0.015%. Determine the probability of finding the following in a mole of acetylene: a. H- 13 C- 13 C-H b. D-...
-
In the neck of the flask depicted in the text, five red balls rest on five blue balls. Suppose the balls are tipped back into the flask, shaken, and the flask is re-inverted. Whats the probability...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


