Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A drying process is used to remove 3000 lbm/h of water (vapor) at steady-state from processed paper. The dryer first heats atmospheric air
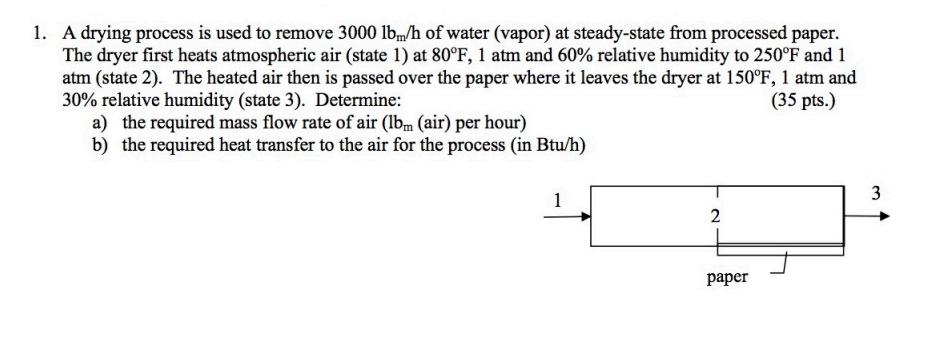


1. A drying process is used to remove 3000 lbm/h of water (vapor) at steady-state from processed paper. The dryer first heats atmospheric air (state 1) at 80F, 1 atm and 60% relative humidity to 250F and 1 atm (state 2). The heated air then is passed over the paper where it leaves the dryer at 150F, 1 atm and 30% relative humidity (state 3). Determine: (35 pts.) a) the required mass flow rate of air (lbm (air) per hour) b) the required heat transfer to the air for the process (in Btu/h) 2 paper 3 2. Gaseous octane (C8H18) enters the combustion chamber of a power plant and burns with 100% theoretical air entering at 25C, 1 atm. The products exit the system at 627C, 1 atm. If the rate of heat transfer from the system to the surroundings is estimated at 15% of the net power developed, determine the molar flowrate of fuel, in kmol/h, for a net power output of 1 MW. (30 pts.) 3. Consider a gas-turbine power plant that operates on an ideal cold-air standard Brayton cycle and delivers an output of 20 MW to the electrical generator. The minimum and maximum cycle temperatures are 520R and 2700R, respectively. The minimum and maximum cycle pressures are 14.0 and 140 psi, respectively. Determine: (35 pts.) a) the power output of the turbine and percent output required to drive the compressor b) the thermal efficiency of the cycle
Step by Step Solution
★★★★★
3.56 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
ANSWER 1 To determine the required mass flow rate of air lbmhr and the required heat transfer to the air Btuhr we can use psychrometric charts and apply the principles of psychrometrics which deal wit...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


