The heat of combustion of butane is 22877 kJ/mol. Use this value to find the heat of
Question:
The heat of combustion of butane is 22877 kJ/mol. Use this value to find the heat of formation of butane. (You may also need to use additional thermochemical data found in Appendix E.)
Data from appendix E
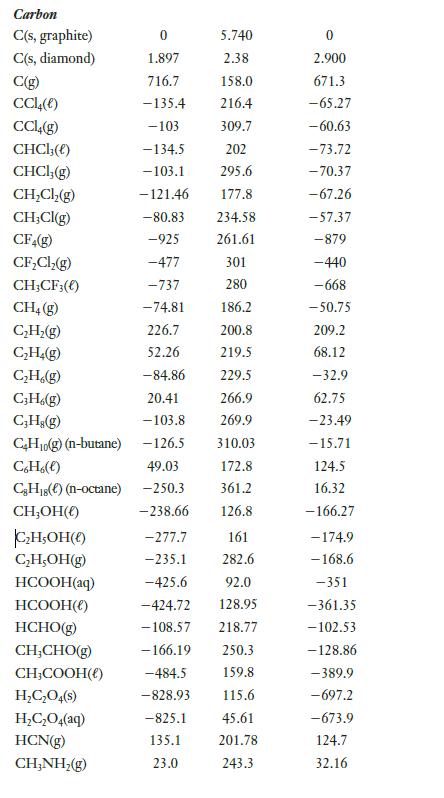
Transcribed Image Text:
Carbon C(s, graphite) C(s, diamond) C(g) CC14(e) CCL4(g) CHCI;(() CHCl, (g) CH₂Cl₂(g) CH₂Cl(g) CF4(g) CF₂Cl₂(g) CH3CF3(0) CH₂(g) C₂H₂(g) C₂H₂(g) C₂H.(g) C3H6(g) C₂H₂(g) C₂H₁0(g) (n-butane) C6H6(e) CH₁8(e) (n-octane) CH₂OH() C₂H5OH() C₂H,OH(g) HCOOH(aq) HCOOH() HCHO(g) CH,CHO(g) CH₂COOH() H₂C₂O4(s) H₂C₂O4(aq) HCN(g) CH,NH,(g) 0 1.897 716.7 -135.4 -103 -134.5 -103.1 - 121.46 -80.83 -925 -477 -737 -74.81 226.7 52.26 5.740 2.38 158.0 216.4 -277.7 -235.1 -425.6 -424.72 -108.57 309.7 202 295.6 177.8 234.58 261.61 301 280 186.2 200.8 219.5 -84.86 229.5 20.41 266.9 -103.8 269.9 -126.5 310.03 49.03 172.8 -250.3 361.2 -238.66 126.8 161 282.6 92.0 128.95 218.77 -166.19 250.3 -484.5 159.8 -828.93. 115.6 -825.1 45.61 135.1 201.78 23.0 243.3 0 2.900 671.3 -65.27 -60.63 -73.72 -70.37 -67.26 -57.37 -879 -440 -668 -50.75 209.2 68.12 -32.9 62.75 -23.49 -15.71 124.5 16.32 -166.27 -174.9 - 168.6 -351 -361.35 -102.53 -128.86 -389.9 -697.2 -673.9 124.7 32.16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)

Answered By

Sandip Nandnawar
I am a B.E (Information technology) from GECA and also have an M.C.M from The University of RTMNU, MH.
I worked as a software developer (Programmer and TL). Also working as an expert for the last 6 years and deal with complex assessment and projects. I have a team and lead a team of experts and conducted primary and secondary research. I am a senior software engg and senior expert and deal with all types of CSE and IT and other IT-related assessments and projects and homework.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The heat of combustion of decahydronaphthalene (C10H18) is -6286 kJ / mol The heat of combustion of naphthalene (C10H8) is-5157 kJ / mol. [In both cases CO2(g) and H2O(l) are the products.] Using...
-
Describe how a promotion discount can communicate information about a product.
-
Nisha has completed her MBA and has joined a company which was going to raise fund from long term sources such as Debt and Equity. Nisha was asked by her manager to prepare a report on which could be...
-
Slapshot Company makes ice hockey sticks. Last week, direct materials (wood, paint, Kevlar, and resin) costing $24,000 were put into production. Direct labor of $40,000 (20 workers x 100 hours x $20...
-
Here are the countries of the top 15 men in the 2013 ING New York City Marathon a. Construct a frequency and relative frequency table of the data. Use the category Other for Ethiopia, South Africa,...
-
Fireside Corporation is organized into four operating segments. The internal reporting system gen erated the following segment information: LO3 Revenues from Intersegment Operating Outsiders...
-
Table P-18 contains data values that represent the monthly sales (in billions of dollars) of all retail stores in the United States. Using the data through 1994, perform a decomposition analysis of...
-
Vertical analysis of balance sheet Balance sheet data for Alvarez Company on December 31, the end of two recent fiscal years, follow: Prepare a comparative balance sheet for both years, stating each...
-
Hydrogen gas will react with either acetylene or ethylene gas. The thermochemical equations for these reactions are provided below. Write the thermochemical equation for the conversion of acetylene...
-
The phase change between graphite and diamond is difficult to observe directly. Both substances can be burned, however. From these equations, calculate H for the conversion of diamond into graphite....
-
Is the set of cash flows depicted below normal or non-normal? Explain. (LG1) Time: 1 2 3 Cash flow -$100 -$50 -$80 50 5 $100 $100
-
The answer above is NOT correct. The value of (2x + 1)(x + x)dx is
-
Review the resource on organizational theory. Explore the various theories and select one to use for this Discussion. Consider the strengths and limitations of the chosen theory. Compose an analysis...
-
How do the locations of Australian department store Myer affect the ability of the other factors of the operating model canvas (suppliers, organization, processes, and information/management systems)...
-
Critical Reading Review: The Exclusion of Latinos from American Media and History Books Read the article. After reading the article, answer the following questions: 1. What purpose do you think the...
-
1. How does the proposed market segment of residential contracts differ from Smith Electric's current market segment? 2.What does a SWOT analysis tell us about Smith Electric's ability to enter a...
-
How do firms report assets on the balance sheet under IFRS?
-
Define the term utility software and give two examples.
-
Two operators can be applied to a function in succession. By definition, AB f(x) = A [B f(x)]. Evaluate AB f(x) if A = d /dx, B = x, and f (x) = cos x.
-
Cos x an eigenfunction of the operator A if A f (x) = xf (x)?
-
Identify the reagents that you would use to accomplish each of the following transformations: (a) (b) H.
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...

Study smarter with the SolutionInn App


