The particulate drawing shown represents an aqueous solution of an acid HA, where A might represent an
Question:
The particulate drawing shown represents an aqueous solution of an acid HA, where A might represent an atom or group of atoms. Is HA a strong acid or a weak acid?
Explain how you can tell from the picture.
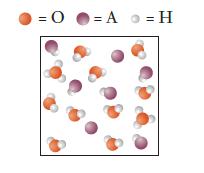
Transcribed Image Text:
= 0 = A = H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
HA is a weak acid be...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
You are given two beakers, one containing an aqueous solution of strong acid (HA) and the other an aqueous solution of weak acid (HB) of the same concentration. Describe how you would compare the...
-
write a java program that create an array of Strings of size n and then check every string if it is pangram or not string is panagram when the string has all alphet english characters Example: input...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
Data obtained from asking the wrong questions at the wrong time or in the wrong place can lead to misleading summary statistics. Explain why the following collection procedures are likely to produce...
-
Recently, the annual inflation rate measured by the Consumer Price Index (CPI) was forecast to be 3.3%. How could a T-bill have had a negative real rate of return over the same period? How could it...
-
For gambling losses, what is the process for determining their deductibility?
-
Give some of the major topics covered in a typical three-week on-site training program for a new aircraft sales representative. What are the three basic ways of compensating salespersons? What is the...
-
Bad Ideas Inc. is the world's only manufacturer of disposable sweaters. After a sweater is made, Bad Ideas can attach buttons on the right, making it suitable for men, or on the left, making it...
-
On December 27, 2018, Glade Company was authorized to issue 10,000 share Surnalize stock issuances for par value preferred stock and 300,000 shares of $22 par value common sa required IL a....
-
Nitric acid (HNO 3 ) can be produced by the reaction of nitrogen dioxide (NO 2 ) and water. Nitric oxide (NO) is also formed as a product. Write a balanced chemical equation for this reaction. Nitric...
-
The picture shown depicts the species present at the start of a combustion reaction between methane, CH 4 , and oxygen, O 2 . (a) Draw the resulting state after this set of reactants has reacted as...
-
Residents in an inner-city area are concerned about drug dealers entering their neighborhood. Over the past 14 nights, they have taken turns watching the street from a darkened apartment. Drug deals...
-
A box is separated by a partition which divides its volume in the ration of 3:1. the larger portion of the box contains 1000 molecules of Ne gas; the smalled portion contains 100 molecules of He gas....
-
The process of translating an idea into goods and services that create value or for which clients will pay is called
-
Let f be twice differentiable with f(0) = 6, f(1) = 8, and f'(1) = 7. Evaluate the following integral. [ = 0 0 xf" (x)dx
-
Although the Chen Company's milling machine is old, it is still in relatively good working order and would last for another 10 years. It is inefficient compared to modern standards, though, and so...
-
PART-3: OFFLINE QUESTIONS - Upload files using the submission link. 1. In 2020 Starbucks began a secret project to develop a competing product against the Keurig Single Serve coffee brewer. The...
-
In the Columbia Gas of Ohio study that forecasted the demand for gas (see p. 155), the company developed the following coefficients for their equation: Growth...
-
Write the given system without the use of matrices. D) - ()- d (x sin t + 8 (2+ 1)
-
Muscalure is the sex pheromone of the common housefly and has the molecular formula C 23 H 46 . When treated with O 3 followed by DMS, the following two compounds are produced. Draw two possible...
-
Propose a plausible mechanism for each of the following reactions: a. b. stitl. [H,SO,] Conc. H2SO4
-
Suggest an efficient synthesis for the following transformation: CI H
-
The stock of Golden Technologies ltd.is currently quoting at Rs. 80 per share in the market. The expected stock price for the next year is as follows: Probability 0.20 0.50 0.20 0.10 Price 120 140...
-
McQueen Car Store has a cost of equity of 10.2 percent. The company has an aftertax cost of debt of 6.0 percent. If the company's debt-equity ratio is .65, what is the weighted average cost of...
-
Simpson's Corporatiocoering a proj hsthlowngshw data Wrecs IRR? Year Cash flows: $1,400$425$425$425 3 O a.-4.44% Ob-4.04% Og-561% O d.-4.53% Oe-4.17%

Study smarter with the SolutionInn App


