The visible lines in the hydrogen atom emission spectrum arise from transitions with a final state with
Question:
The visible lines in the hydrogen atom emission spectrum arise from transitions with a final state with n = 2. In what spectral region should we expect to find transitions that have a final state of n = 1? Explain your reasoning using an energy level diagram similar to the one in Problem 6.26.
Data from problem 6.26
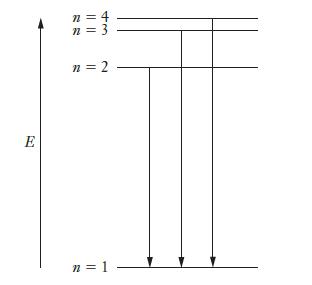
The figure below depicts the first four energy levels in a hydrogen atom. The three transitions shown as arrows emit ultraviolet light and occur at wavelengths of 121.566 nm, 102.583 nm, and 97.524 nm, respectively. Find the frequency of light that would be emitted in a transition from the state labeled as n = 4 to the state labeled as n = 3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





