Use information from Section 6.7 to estimate which form of electromagnetic radiation is the lowest energy ionizing
Question:
Use information from Section 6.7 to estimate which form of electromagnetic radiation is the lowest energy ionizing radiation.
Data from section 6.7


Transcribed Image Text:
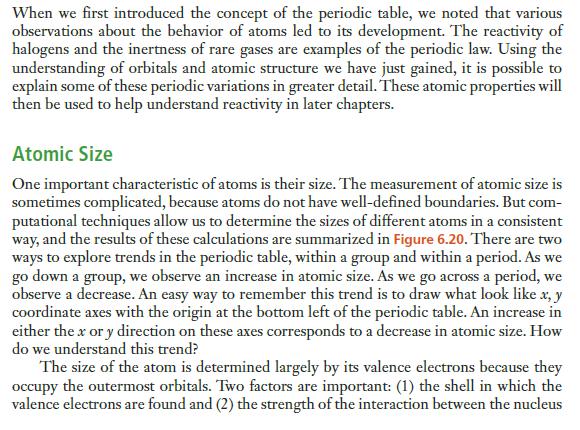
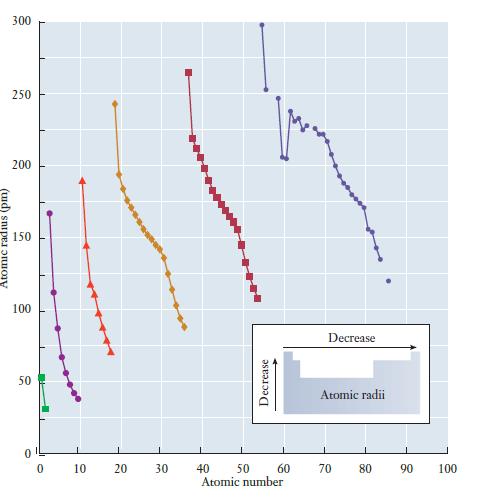
When we first introduced the concept of the periodic table, we noted that various observations about the behavior of atoms led to its development. The reactivity of halogens and the inertness of rare gases are examples of the periodic law. Using the understanding of orbitals and atomic structure we have just gained, it is possible to explain some of these periodic variations in greater detail. These atomic properties will then be used to help understand reactivity in later chapters. Atomic Size One important characteristic of atoms is their size. The measurement of atomic size is sometimes complicated, because atoms do not have well-defined boundaries. But com- putational techniques allow us to determine the sizes of different atoms in a consistent way, and the results of these calculations are summarized in Figure 6.20. There are two ways to explore trends in the periodic table, within a group and within a period. As we go down a group, we observe an increase in atomic size. As we go across a period, we observe a decrease. An easy way to remember this trend is to draw what look like x, y coordinate axes with the origin at the bottom left of the periodic table. An increase in either the x or y direction on these axes corresponds to a decrease in atomic size. How do we understand this trend? The size of the atom is determined largely by its valence electrons because they occupy the outermost orbitals. Two factors are important: (1) the shell in which the valence electrons are found and (2) the strength of the interaction between the nucleus
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
In Section 67 its typically stated that ionizing radiation includes gamma rays Xrays and certain ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
How do organizations reconcile the tension between the pursuit of workforce diversity and the imperative to maintain cohesion and alignment within teams, particularly in contexts where diverse...
-
Developments in Technology Light is incident from air on the end face of a multimode optical fibre at angle of incidence as shown below. n n 1 2 The refractive indices of the core and cladding are...
-
The energy from radiation can be used to cause the rupture of chemical bonds. A minimum energy of 941 KJ/mol is required to break the nitrogen-nitrogen bond in N2. What is the longest wavelength of...
-
10 1 Journalize the following transactions for Bigelow Company for the month of October. 31 Oct Stockholders invest cash in the company in exchange for common stock. 5 Oct The company buys a delivery...
-
Record the following into the general journal of Rick's Auto Shop. 201X Apr. 1 Rick Savareses invested $80,000 cash in the auto shop. 5 Paid $15,000 for auto equipment. 8 Bought auto equipment from...
-
Assume that Jaciel has been working for the same fi rm for 25 years. Over the last 5 years, her salary has increased by $1,000 each year from $41,000 to $45,000. The average salary of her fi nal 5...
-
From your own working experience what examples have you seen of evangelisation, parables and apostles as methods of coordination?? LO1
-
Healthy Potions, Inc., is considering investing in a new production line for eye drops. Other than investing in the equipment, the company needs to increase its cash and cash equivalents by $10,000,...
-
The status of all job activity occurring during January for XYZ Manufacturing is shown below: Job # X-1 X-2 X-3 X-4 Beginning WIP Inventory $0 $15,000 $0 $26,000 January Direct costs incurred: Direct...
-
A piece of cloth is discovered in a burial pit in the southwestern United States. A tiny sample of the cloth is burned to CO 2 , and the 14 C/ 12 C ratio is 0.250 times the ratio in todays...
-
How does make determine whether a target needs to be rebuilt?
-
Tara buys only shoes and socks. When the price of shoes goes up, Tara continues buying exactly the same number of socks as before. True or False: Socks could not possibly be an inferior good for Tara.
-
Write a brief statement that interprets the confidence interval. Choose the correct answer below. A. There is a 99% chance that the true value of the population mean weight of newborn girls will fall...
-
Transcribed image text: If estimated annual factory overhead is $1,072,500; overhead is applied using direct labor hours, estimated annual direct labor hours are 275,000 actum March factory overhead...
-
Your firm has limited capital to invest and is therefore interested in comparing projects based on the profitability index (PI), as well as other measures. What is the PI of the project with the...
-
The following rates are applicable to annual payroll in British Columbia Question 17 options: 1234 1.95% x total B.C. remuneration 1234 2.925% x (B.C. remuneration - $500,000) 1234 Tax Rate 1234...
-
Assume that different groups of couples use a particular method of gender selection and each couple gives birth to one baby. This method is designed to increase the likelihood that each baby will be...
-
Farmer Tom Hedges anticipates taking 100,000 bushels of oats to the market in three months. The current cash price for oats is $2.15. He can sell a three-month futures contract for oats at $2.20. He...
-
This problem continues the Draper Consulting, Inc., situation from Problem 12-45 of Chapter 12. In October, Draper has the following transactions related to its common shares: Oct 1 Draper...
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


