Which of the following is more likely to precipitate sulfate ions? PbSO4(s) Pb+ (aq) + SO4 (aq)
Question:
Which of the following is more likely to precipitate sulfate ions?
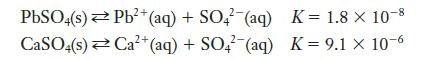
Transcribed Image Text:
PbSO4(s) Pb²+ (aq) + SO4 (aq) 2+ CaSO4(s) Ca²+ (aq) + SO4 (aq) K = 1.8 X 10-8 K = 9.1 x 10-6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Lead is m...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
In Example Problem 12.2, we saw that hydroxide ions precipitate with calcium. Magnesium ions show similar behavior. The two pertinent equilibria are: Which ion is more likely to precipitate hydroxide...
-
Which of the following is more likely to precipitate the hydroxide ion? Cu(OH) (s) Ca(OH)(s) 2+ Cu+ (aq) + 2 OH(aq) Ca+ (aq) + 2 OH- (aq) K 1.6 X 10-19 K = 7.9 x 10-6
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
paraphrase the following two passages using narrative in-text citations: 1.Those who teach ethics don't need to look far for lessons. Every day there's fresh scandal: Google is in hot water for how...
-
Is Samsungs product development process customer centered? Team based? Systematic? In the world of consumer electronics, copycat brands are a dime a dozen. These are the brands consumers turn to if...
-
Using a graphing calculator, find the real zeros of the function. Approximate the zeros to three decimal places. a) f (x) = x3 - 3x - 1 b) f (x) = x3 + 3x2 - 9x - 13 c) f (x) = x4 - 2x2
-
Maximum use of space. Usually the largest capital cost is for space. This means not only floor space but cubic space as well since goods are stored in the space above the floor as well as on it. LO.1
-
Jessica Company manufactures hockey pucks and soccer balls. For both products, materials are added at the beginning of the production process and conversion costs are incurred evenly. Jessica uses...
-
Capital Rationing Decision for a Service Company Involving Four Proposals Clearcast Communications Inc. is considering allocating a limited amount of capital investment funds among four proposals....
-
Write equilibrium expressions for each of the following heterogeneous equilibria. (a) CaCO 3 (s) Ca 2+ (aq) + CO 3 2 (aq) (b) AgCl(s) Ag + (aq) + Cl (aq) (c) Mg 3 (PO 4 ) 2 (s) 3 Mg 2+ (aq) + 2...
-
What is the pH of 0.10 M NaOH? Strategy We know that NaOH is a strong base, so it will completely dissociate in solution. This gives us [OH ]. We can use the K w relationship between [OH ] and [H 3...
-
a. Show that the capacitance C of an isolated conducting sphere of radius r is given by the formula: C = 4 0 r This diagram shows two identical conducting brass spheres of radius 10Cm mounted on...
-
Sketch the curves with equations given in question 3 parts a, b, c and d, labelling any stationary points with their coordinates. Data from Question 3 Find the coordinates of the points where the...
-
Trace the polygon and point P on paper. Then draw a rotation of the polygon the given number of degrees about P. 130 Q R T P S
-
Name any devices other than the three mentioned in Section 31. 1-battery, solar cell, and electric generator that can act as a power source in an electric circuit. Data from Section 31. 1...
-
A thick resistor and a thin resistor of the same length and material are connected in series, as shown in Figure 31. 29. Which resistor has \((a)\) the greater potential difference across it and...
-
Note that the amount of 25k that has already been spent on developing the website is not included in this analysis, as it represents a sunk cost. The decision rule for ARR is that a project should be...
-
HP has been engaged in environmental matters for some years, how might this help them to be more effective at it?
-
What is removed during each of the three stages of wastewater treatment: primary, secondary, and tertiary? During which state would you expect items to be recovered that were accidentally flushed,...
-
Data taken from a stressstrain test for a ceramic are given in the table. The curve is linear between the origin and the first point. Plot the diagram, and determine the modulus of elasticity and the...
-
Data taken from a stressstrain test for a ceramic are given in the table. The curve is linear between the origin and the first point. Plot the diagram, and determine approximately the modulus of...
-
The stressstrain diagram for a steel alloy having an original diameter of 0.5 in. and a gage length of 2 in. is given in the figure. Determine approximately the modulus of elasticity for the...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


