A balloon filled with oxygen gas at 25 C occupies a volume of 2.1 L. Assuming that
Question:
A balloon filled with oxygen gas at 25 °C occupies a volume of 2.1 L. Assuming that the pressure remains constant, what is the volume at 100 °C?
Strategy
Convert the temperatures to kelvins and use Equation 6.2.
Equation 6.2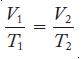
Transcribed Image Text:
V₁ E T₁ V2 T₂ 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
List the data given by the problem Rearrange the equation to p...View the full answer

Answered By

Ajay Negi
Hi, I've completed my degree in engineering (Information Technology) from an NIT. Currently working as a software engineer. Wish to impart quality education to the future generation.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
i) ii) What is the irradiance (in W/m) that falls onto a surface that is positioned perpendicularly to the LED, as shown in the figure below, for a) r = 0 and b) an arbitrary value of r? [6 points]...
-
A balloon filled with 39.1 moles of helium has a volume of 876 L at 0.08C and 1.00 atm pressure. At constant pressure, the temperature of the balloon is increased to 38.08C, causing the balloon to...
-
The small bubbles that form on the bottom of a water pot that is being heated (before boiling) are due to dissolved air coming out of solution. Use Henrys law and the solubilities given to calculate...
-
Suppose \(x\) is a linked-list Node. What is the effect of the following code fragment? \[x \cdot \text { next }=x \cdot \text { next } . \text { next; }\]
-
Use Equation 17-13 to calculate the de Broglie wavelength for an electron of kinetic energy (a) 2.5eV, (b) 250eV, (c) 2.5keV, and (d) 25keV. 1.23 (K in electron volts) 17-13 VK
-
(a) An object of proper length Lp takes a time interval t to pass an Earth observer. Determine the speed of the object as measured by the Earth observer. (b) A column of tanks, 300 m long, takes 75.0...
-
Formulate potential hypotheses regarding the likely impact of your sports teams win or loss on your feelings toward them and your purchase intentions.
-
The wedding date for a couple is quickly approaching, and the wedding planner must provide the caterer an estimate of how many people will attend the reception so that the appropriate quantity of...
-
Why is it important to a firm to be able to produce Responsibility Accounting Reports for cost and profits centers?
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,500 cases of Oktoberfest-style beer from a German supplier for 390,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Make a drawing of an open-end manometer measuring the pressure of a sample of a gas that is at 200 torr. Assume the pressure on the open end is 1.00 atm.
-
Th e volume of a sample of nitrogen gas increases from 0.440 L at 27 C to 1.01 L as it is heated to a new temperature. Calculate the new temperature of the nitrogen.
-
A total of 803 adults were asked whether they prefer to drink coffee, tea, or neither. Table 14 compares the adults responses and the regions of the country where they live. Let S stand for a person...
-
Why do you think it is important to consider only relevant costs when conducting a differential analysis for a major purchase? Why not consider all possible costs in your decision? provide specific...
-
How do power dynamics and influence tactics shape decision-making processes and organizational politics within hierarchical structures ?
-
How do I answer these given the information below? Loan Amount? Loan to Value? Loan to Cost? Payment amount? Loan Balance at Maturity? Given Information: Property Cost: $1,000,000 Bank Policy on LTV:...
-
In your initial post, first do the following: Use scholarly references to define Project Management (PM), Systems Development Life Cycle (SDLC), and Application Life Cycle (AL). Then, in the same...
-
How do concepts of diversity and inclusion vary across different cultural and geographical contexts, and what strategies can multinational organizations employ to navigate these variations...
-
FCC metals are often recommended for use at low temperatures, particularly when any sudden loading of the part is expected. Explain?
-
A sample statistic will not change from sample to sample. Determine whether the statement is true or false. If it is false, rewrite it as a true statement.
-
Arrange the following in terms of decreasing bond energy and bond length: O + 2 , O 2 , O - 2 , and O 2- 2 .
-
Images of molecular orbitals for LiH calculated using the minimal basis set are shown here. In these images, the smaller atom is H. The H1s AO has a lower energy than the Li2s AO. The energy of the...
-
What is the electron configuration corresponding to O 2 , O 2 , and O + 2 ? What do you expect the relative order of bond strength to be for these species? Which, if any, have unpaired electrons?
-
[ The following information applies to the questions displayed below ] Nauticat has two classes of stock authorized: $ 1 0 par preferred, and $ 1 par value common. As of the beginning of 2 0 2 1 , 1...
-
Selling is not the most important part of marketing. Explain why not
-
When direct materials are issued from the storeroom, are any entries made in the subsidiary records? Question 2 options: Increase raw material item record Decrease raw material item record No entry...

Study smarter with the SolutionInn App


