A pipet was used to measure 10.00 mL of a sulfuric acid solution into a titration flask.
Question:
A pipet was used to measure 10.00 mL of a sulfuric acid solution into a titration flask. It took 31.77 mL of 0.102 M NaOH to neutralize the sulfuric acid completely. Calculate the concentration of the sulfuric acid solution. Assume that the reaction is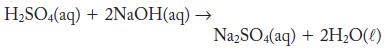
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Preparation of Page One of Form 1120 Complete the header section to include company information, with no errors. Consider the following as you fill out this section of the form: Significance of the...
-
Procedures Experiment 1: Standardize the Iodine Solution Part 1: Prepare the Materials Take a 100 mL volumetric flask from the Containers shelf and place it on the workbench. Take ascorbic acid from...
-
Procedures Experiment 1: Standardize the Iodine Solution Part 1: Prepare the Materials Take a 100 mL volumetric flask from the Containers shelf and place it on the workbench. Take ascorbic acid from...
-
All else equal, a large sell off in the equity markets (decline in the S&P 500 and the Dow Jones Industrial Average) would be the most likely result from the reporting of a much higher than expected...
-
Show the products you would obtain by reduction of the following esters withLiAlH4: (b) (a) CHH2CH2
-
A furniture manufacturer has warehouses in cities represented by nodes 1, 2, and 3 in Figure. The values on the arcs indicate the per unit shipping costs required to transport living room suites...
-
What ethical guidelines might you propose for strategic decision makers when doing competitor Intelligence? (R-56)
-
Your supervising attorney recalls that there was a 1989 Arkansas case in which a county court judge was convicted in federal court of vote buying. After the United States Court of Appeals denied the...
-
Question 18 3.33 pts The following information describes a company's usage of direct labor in a recent period: Actual hours used 23,500 Actual rate per hour $15 Standard rate per hour $14 Standard...
-
You have a pH buffer made from 0.010 M acetic acid and 0.020 M sodium acetate. To buffer a biological reaction, you add 5.0 mL of this buffer to 1.0 L of a solution that contains the system of...
-
Does adding the second compound increase, decrease, or have no effect on the solubility of the first compound? (a) Aluminum hydroxide and NaOH (b) Magnesium phosphate and HNO 3
-
Theres an IKEA TV ad that features a discarded lamp, forsaken on a rainy night in some American city. A man looks at the camera and says in a sympathetic Swedish accent, Many of you feel bad for this...
-
Hackett Produce Supply is preparing its cash budget for April. The following information is available: Estimated credit sales for April Actual credit sales for March Estimated collections in April...
-
006 10.0 points A pendulum clock was moved from a location where g = 9.8168 m/s to another location where 9 9.806 m/s. During the move, g = the length of the clock's pendulum did not change;...
-
6-3x 2 Problem 6. (a) Find L So 6-3x-2y 3 2 dz dy dx. (b) Find the limits of integration. No need to find the integral. dx dz dy. Hint: The plane in the image is given by 3x + 2y + 3z = 6. 2.0 1.5...
-
Question Under what scale of measurement(s) can we say that : Jim weighs 4X as much as Edie? Sam is heavier than Sue? Jim and Sam don't weigh the same? Jim is much heavier than Sam, but Mary is only...
-
Lender Company provides postretirement health care benefits to employees who provide at least 10 years of service and reach the age of 65 while in service. On January 1 of the current calendar year,...
-
Imagine a container placed in a tub of water, as depicted in the accompanying diagram. (a) If the contents of the container are the system and heat is able to flow through the container walls, what...
-
Calculate the Lagrange polynomial P 2 (x) for the values (1.00) = 1.0000, (1.02) = 0.9888, (1.04) = 0.9784 of the gamma function [(24) in App. A3.1] and from it approximations of (1.01) and (1.03).
-
A 3-in plastic butterfly valve carries 300 gal/min of gasoline at 77F. Compute the expected pressure drop across the valve.
-
A 10-in plastic butterfly valve carries 5000 gal/min of liquid propane at 77F. Compute the expected pressure drop across the valve.
-
A 1-in plastic diaphragm valve carries 60 gal/min of carbon tetrachloride at 77F. Compute the expected pressure drop across the valve.
-
Estimate the intrinsic value of the stock company ABC. Dividends were just paid at $8 per share and are expected to grow by 5%. You require 20% on this stock given its volatile characteristics. If...
-
Crane, Inc., a resort management company, is refurbishing one of its hotels at a cost of $6,794,207. Management expects that this will lead to additional cash flows of $1,560,000 for the next six...
-
Match each of the following transactions with the applicable internal control principle that is being violated

Study smarter with the SolutionInn App


