Aspirin is a remarkable analgesic (painkiller) that also appears to prevent certain heart conditions. From its chemical
Question:
Aspirin is a remarkable analgesic (painkiller) that also appears to prevent certain heart conditions. From its chemical formula, C9H8O4, calculate the percentage by mass of each element in aspirin.
Strategy
A flow diagram outlines the strategy for this problem. Th e formula is used to calculate the masses of each element in the compound, and those numbers are used to calculate the percentage composition.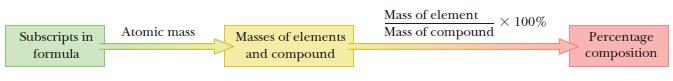
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





