Assign the oxidation numbers of all atoms in the following species.
Question:
Assign the oxidation numbers of all atoms in the following species.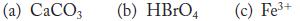
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
When assigning oxidation numbers to the atoms in a compound we follow a set of rules that help us de...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Assign the oxidation numbers of all atoms in the following species.
-
Assign the oxidation numbers of all atoms in the following compounds.
-
Assign the oxidation numbers of all atoms in the following compounds. (a) KHF 2 (b)H 2 Se (c)NaO 2 (d)C 2 H 6
-
Quality Air Conditioning manufactures three home air conditioners: an economy model, a standard model, and a deluxe model. The profits per unit are $63, $95, and $135, respectively. The production...
-
One scheme for extending the operation of gas turbine blades to higher temperatures involves applying a ceramic coating to the surfaces of blades fabricated from a super alloy such as inconel. To...
-
Identify and describe the different schedules for delivering reinforcement. What should managers consider most when deciding which schedule to use?
-
(Horizontal and Vertical Analysis) Presented below are comparative balance sheets for the Eola Yevette Company. Instructions (a) Prepare a comparative balance sheet of Yevette Company showing the...
-
Iacouva Company reported the following on the companys income statement for 2014 and 2013: a. Determine the number of times interest charges are earned for 2014 and 2013. Round to one decimal place....
-
D&L Corporation's profit and loss statement showed a net income of 93/4% of revenue or $41,250. Twenty-one percent of net income was paid in corporation tax and 65% of the net income after tax was...
-
Balance the following reactions, and specify which species is oxidized and which is reduced. (a) H 2 + O 2 H 2 O (b) Fe + O 2 Fe 2 O 3 (c) Al 2 O 3 + C Al + CO 2
-
Assign the oxidation numbers of all atoms in the following species.
-
Workers in a certain job are trained by the company, and the company calculates that to recoup its investment costs, the workers wages must be $5 per hour below their marginal productivity. Suppose...
-
Which of the five hazardous attitudes do you display most frequently? What can you do to minimize the presence and impact of these attitudes in your life?
-
What brought you to this course? How do you define Black or Blackness? What do you hope to get out of this class? When you think of Black Culture, what is the first thing that comes to mind? [For...
-
1. What is XBRL Taxonomy? How do you as a preparer of financial statement use the XBRL Taxonomy in locating a label for a specific financial element? 2. What are the benefits of adopting XBRL from...
-
What a business can do to protect and minimize the invasion of privacy for their customers? Think of your experience when visiting a website. What do most websites have you agree to before you do...
-
Based on your interest, skill set, or goals what do you typically contribute when working in groups? What do you need others to contribute due to your lack of interest, skill set, or goals? How do...
-
(a) What is the octet rule? (b) How many electrons must a sulfur atom gain to achieve an octet in its valence shell? (c) If an atom has the electron configuration 1s22s22p3, how many electrons must...
-
Gordon and Lisa estimate that they will need $1,875,000 in 40 years for their retirement years. If they can earn 8 percent annually on their funds, how much do they need to save annually?
-
Two mischievous children drop water balloons from a bridge as depicted in Figure P3.87. Each water balloon is approximately 30 cm in diameter, and in this figure the red balloon is about 1.8 m below...
-
An impish young lad stands on a bridge 10 m above a lake and drops a water balloon on a boat of unsuspecting tourists. Although the boat is traveling at a speed of 7.5 m/s, the boy manages to land...
-
A boy pushes a 3.1-kg book against a vertical wall with a horizontal force of 40 N. What is the minimum coefficient of friction that will keep the book in place without sliding?
-
THIS IS ONE QUESTION WITH TWO PARTS. PLEASE ANSWER COMPLETELY AND SHOW ALL WORK. (NO EXCEL) Information for Question 1: State Probability Retum on A Return on B Return on C Retum on Portfolio X Boom...
-
Direct materials (5.0 Ibs. @ $5.00 per Ib.) Direct labor (2.0 hrs. @ $13.00 per hr.) Overhead (2.0 hrs. @ $18.50 per hr.) Total standard cost $25.00 26.00 37.00 $88.00 The predetermined overhead rate...
-
Problem 1-28 (Algo) (LO 1-4, 1-5, 1-6b 1-7) Harper, Inc., acquires 40 percent of the outstanding voting stock of Kinman Company on January 1, 2020, for $316,100 in cash. The book value of Kinman's...

Study smarter with the SolutionInn App


