Balance each of the following redox reactions in basic solution.
Question:
Balance each of the following redox reactions in basic solution.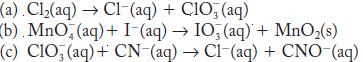
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To balance the redox reactions in a basic solution you need to follow several steps First identify the oxidation states of the elements and separate t...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following redox reactions in basic solution.
-
Balance each of the following redox reactions in basic solution.
-
Balance each of the following redox reactions in basic solution.
-
Suppose you are going to put up your own company, list down at least ten of your possible contributions to the socio-economic development in your community. Name of Business: Name of Owner/s: 2. 4....
-
The absorber plate of a solar collector may be coated with an opaque material for which the spectral, directional absorptivity is characterized by relations of the form the zenith angle is formed by...
-
Implement the following LP model in a spreadsheet. Use Solver to solve the problem and create a Sensitivity Report. Use this information to answer the following questions: MAX: 2X1 + 4X2 Subject to:...
-
Use a worksheet for employers pension plan entries.
-
The 2014 accounting records of Rogan Transport reveal these transactions and events. Instructions Prepare the cash flows from operating activities section using the directmethod. Payment of interest...
-
March sales are $100,000 and cost of goods sold is $65,000. What is the entry to Finished Goods Inventory? Question 5 options: $65,000 debit $65,000 credit $35,000 debit $100,000 credit
-
Why is the following balanced reaction not a proper redox reaction?
-
Balance each of the following redox reactions in acid solution.
-
What aspect of the history of foodservice surprises you? LO1
-
what extent do you perceive that your personal values align with the core ethos and culture of the organization?
-
Safe, avoidant, indecisive, and disorganized. What attachment style do you believe you grew up with, and how did it affect your cognitive and personality development as a child? Think about the types...
-
What do you think about an 'employee-centric' rather than an 'employer-centric' PMS. Which would work better in your current (or prior) organization? Make sure to provide specific examples to justify...
-
How do I relate the below case study to RLR - Responsible Leadership for Relations? Relate and analyses in detail....
-
How do advanced integrative approaches, combining elements of cognitive-behavioral therapy, mindfulness, and somatic experiencing, offer comprehensive solutions for addressing the multifaceted nature...
-
Write the electron configuration for each of the following ions, and determine which ones possess noble-gas configurations: (a) Sr2+ (b) Ti2+ (c) Se2- (d) Ni2+ (e) Br- (f) Mn3+
-
Gopher, Inc. developing its upcoming budgeted Costs of Quality (COQ) with the following information: Expense Item Budget Raw Materials Inspection $ 15,000 EPA Fine 200,000 Design Engineering 15,000...
-
A squirrel is resting in a tall tree when it slips from a branch that is 50 m above the ground. It is a very agile squirrel and manages to land safely on another branch after only 0.50 s. What is the...
-
Basketball on the Moon. If LeBron James can jump 1.5 m high on Earth, how high could he jump on the Moon (assume an indoor court), where g - 1.6 m/s 2 ?
-
An apple falls from a branch near the top of a tall tree. If the branch is 12 m above the ground, what is the apples speed just before it hits the ground?
-
When direct materials are issued from the storeroom, are any entries made in the subsidiary records? Question 2 options: Increase raw material item record Decrease raw material item record No entry...
-
Riverrun Co. provides medical care and insurance benefits to its retirees. In the current year, Riverrun agrees to pay $5,500 for medical insurance and contribute an additional $9,000 to a retirement...
-
DETAILS 1. [-/1 Points) SMITHNM13 11.2.025. MY NOTES Convert the credit card rate to the APR. Oregon, 2% per month % Need Help? ReadIt Watch

Study smarter with the SolutionInn App


