Balance each of the following redox reactions in acid solution.
Question:
Balance each of the following redox reactions in acid solution.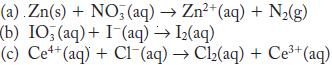
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a 2A1s 3C10aq 3H2Ol 20Haq ...View the full answer

Answered By

Rishabh Ojha
During my undergraduate i used to participate as TA (Teaching Assistant) in several electronics and computers subject. I'm passionate about learning Computer Science as my bachelors are in Electronics but i learnt most of the Computer Science subjects on my own which Machine Learning also. At Present, i'm a working professional pursuing my career as a Machine Learning Engineer and i want to help others learn during my free hours, that's all the motivation behind giving tuition. To be frank i have no prior experience of tutoring but i have solved problems on opensource platforms like StackOverflow and github. ~Thanks
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following redox reactions in acid solution.
-
Balance each of the following redox reactions in acid solution.
-
Balance each of the following redox reactions in acid solution.
-
Single PlantwideandMultiple Production Department Factory Overhead Rate Methodsand Product Cost Distortion The management of Nova Industries Inc. manufactures gasoline and diesel engines through two...
-
Consider the evacuated tube solar collector described in part (d). In the interest of maximizing collector efficiency, what spectral radiative characteristics are desired for the outer tube and for...
-
Consider the following constraint, where S is a slack variable: 2X1 + 4X2 + S = 16 a. What was the original constraint before the slack variable was included? b. What value of S is associated with...
-
Identify types of pension plans and their characteristics.
-
The trial balance of Sam Mitchell, CPA, is dated January 31, 2015: During February, the business completed the following transactions: Feb. 4 Collected $ 4,000 cash from a client on account. 8...
-
Webster Corperation's monthly projected general and administrative expenses include $5,000 administrative salaries, $2,400 of other cash administrative expenses, $1,350 of depreciation expense, and...
-
Balance each of the following redox reactions in basic solution.
-
Complete and balance each half-reaction in acid solution, and identify it as an oxidation or a reduction.
-
Compute the mean of the numbers \(12,15,17,18,18\), and 19.
-
Imagine that you are faced with three alternative projects, each of which costs $1 Million (M). Assuming the discount rate of 10 percent and efficiency is your only criterion, which one of these...
-
imagine that you are the Hotel General Manager of a hotel that you helped to design. Answer the following questions: Your role as the Hotel General Manager As the Hotel General Manager, describe your...
-
What types of problems may a multinational corporation face? What inconsistencies may multinational corporations face as a result of differences in cultures and values? What practices pose ethical...
-
What is a view? What is a viewpoint? What is a visualization? How do they come together to help communicate a model to the stakeholders?
-
A spray dryer receives distillers dried grains (DDGS) with a 65% moisture content. The spray dryer operates with dry air entering at 180 C, 1 bar, and moist air exits at 87 C, 1 bar, and 25% relative...
-
(a) Write the electron configuration for the element titanium, Ti. How many valence electrons does this atom possess? (b) Hafnium, Hf, is also found in group 4B. Write the electron configuration for...
-
Big Jim Company sponsored a picnic for employees and purchased a propane grill equipped with a standard-sized propane tank for the picnic. To make sure there was enough propane for all the cooking...
-
You are standing across the street from a tall building when the top of the building (h = 80 m) is hit by lightning and a brick is knocked loose. You see the lightning strike and immediately see that...
-
A cable attached to a block of mass 12 kg pulls the block along a horizontal floor at a constant velocity. If the tension in the cable is 5.0 N, what is the coefficient of kinetic friction between...
-
A crate of mass 55 kg is attached to one end of a string, and the other end of the string runs over a pulley and is held by a person as in Figure 3.22A. If the person pulls with a force of 85 N, what...
-
Los siguientes datos corresponden a las operaciones de Turk Company el ao pasado: Ventas $ 900 000 Utilidad operativa neta $ 36 000 Margen de contribucin $ 150 000 Activos operativos promedio $ 180...
-
Problem 16-16 Tax Shields (LO2) River Cruises is all-equity-financed with 53,000 shares. It now proposes to issue $280,000 of debt at an interest rate of 12% and to use the proceeds to repurchase...
-
In a process costing system, companies use predetermined overhead rates to apply overhead

Study smarter with the SolutionInn App


