Balance each of the following redox reactions in acid solution.
Question:
Balance each of the following redox reactions in acid solution.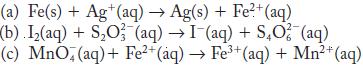
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To balance redox reactions in an acid solution we need to follow certain steps Lets go through each of the reactions one by one Reaction a Fes Agaq Ag...View the full answer

Answered By

Saud Ur Rehman
Evaluating manufacturing processes by designing and conducting research programs; applying knowledge of product design, fabrication, assembly, tooling, and materials; conferring with equipment vendors; soliciting observations from operators. Developing manufacturing processes by studying product requirements; researching, designing, modifying, and testing manufacturing methods and equipment; conferring with equipment vendors. Keeping equipment operational by coordinating maintenance and repair services; following manufacturer's instructions and established procedures; requesting special service.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following redox reactions in acid solution.
-
Balance each of the following redox reactions in acid solution.
-
Balance each of the following redox reactions in acid solution.
-
The Illinois State University Alumni Association is having two Baseball Day events this year. The purpose of the events is to both foster relationships between ISU alums and to also raise money for...
-
A deep cavity of 50-mm diameter approximates a blackbody and is maintained at 250C while exposed to solar irradiation of 800 W/m 2 and surroundings and ambient air at 25C. A thin window of spectral...
-
The text compares the failure rate for small businesses with the divorce rate in marriage and the student failure rate in college. Are these fair comparisons?
-
Distinguish between accounting for the employers pension plan and accounting for the pension fund.
-
Two pendulums have the same dimensions (length L) and total mass (m). Pendulum A is a very small ball swinging at the end of a uniform mass less bar. In pendulum B, half the mass is in the ball and...
-
Haas Company manufactures and sells one product. The following information pertains to each of the company's first three years of operations: 1 0 points e B o o k Hint Ask References Variabte costs...
-
Balance each of the following redox reactions in basic solution.
-
Complete and balance each half-reaction in acid solution, and identify it as an oxidation or a reduction.
-
A hedge fund charges an incentive fee of 20% of any investment returns above the T-bill rate, which currently is 2%. In the first year, the fund suffers a loss of 8%. What rate of return must it earn...
-
Lifestyle is how one enacts the self-concept. The way they would enact it is through buying luxury items which is the most premium iPhone. The latent reasons why people want an iPhone 15 all have to...
-
Make a Tows Matrix that assess the strengths, weakness, opportunities, and threats for Dannon based on the case study For typical corporate strategies under purpose of communication. Strengths 1) 2)...
-
Now that you've watched the lectures, The Abilene Paradox movie, and the Challenger Disaster Video, I'd like you to think for a moment about when you may have observed the Abilene Paradox or...
-
Ensuring that the projectadheres to the selected quality standard . Often, ensuring that the project work is done 'correctly' is as important as ensuring that the end result fulfills the project's...
-
Think about some career planning and development issues; for example, mergers and reorganization uncertainty, lack of upward mobility, getting managers to understand your career potential, and...
-
Average bond enthalpies are generally defined for gas-phase molecules. Many substances are liquids in their standard state. By using appropriate thermo-chemical data from Appendix C, calculate...
-
A genetically engineered strain of Escherichia coli (E. coli) is used to synthesize human insulin for people suffering from type I diabetes mellitus. In the following simplified reaction scheme,...
-
A car of mass 1200 kg is lowered onto a junk pile at the end of the cable of a large crane. If the car is accelerating downward at 0.20 m/s 2 , what is the tension in the cable?
-
You work for a moving company and are given the job of pulling two large boxes of mass m 1 = 120 kg and m 2 = 290 kg using ropes as shown in Figure P3.63. You pull very hard, and the boxes are...
-
Give an example of motion in which the acceleration and the velocity are in opposite directions.
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


