Balance each of the following redox reactions in acid solution.
Question:
Balance each of the following redox reactions in acid solution.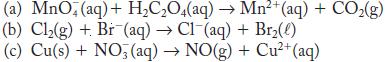
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
a Fes 2Agaq 2Ags F...View the full answer

Answered By

Munibah Munir
I've done MS specialization in finance’s have command on accounting and financial management. Forecasting and Financial Statement Analysis is basic field of my specialization. On many firms I have done real base projects in financial management field special forecasting. I have served more than 500 Clients for more than 800 business projects, and I have got a very high repute in providing highly professional and quality services.I have capability of performing extra-ordinarily well in limited time and at reasonable fee. My clients are guaranteed full satisfaction and I make things easy for them. I am capable of handling complex issues in the mentioned areas and never let my clients down.
4.60+
467+ Reviews
648+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following redox reactions in acid solution.
-
Balance each of the following redox reactions in acid solution.
-
Balance each of the following redox reactions in acid solution.
-
Israel We I had been in Israel for two weeks. We were in our church tour bus, late at night, driving through the mountainous desert. The night was black no moon and only a few stars. The only light...
-
(a) Estimate the roof temperature under steady-state conditions. (b) Explore the effect of changes in the absorptivity, emissivity, and convection coefficient on the steady-state temperature.
-
Describe the significance of triggering events in entrepreneurship. Give examples.
-
Powerpuff Corp. carries an account in its general ledger called Investments, which contained the following debits for investment purchases and no credits (a) Assuming that all the securities are...
-
Stacey Small has a small salon that she has run for a few years as a sole proprietorship. The proprietorship uses the cash method of accounting and the calendar year as its tax year. Stacey needs...
-
Following your advice, Peet Ltd. has entered into an FRA agreement with Alpha Ltd. in which Peet Ltd. borrows $ 200,000,000 in 3-month time for a period of 6 months, and Alpha Ltd. invests $...
-
Balance each of the following redox reactions in basic solution.
-
Complete and balance each half-reaction in acid solution, and identify it as an oxidation or a reduction.
-
The owner of an apartment building can rent all 50 apartments if she charges $1800 per month, but she rents one fewer apartment for each $60 increase in monthly rent. (a) Construct a table that gives...
-
Essay on: The Influence of Social Media on Individuals, Family, and Society. Also how social media influences us on a business end, how it influences us personally, and how they correlate.
-
A wastewater flow of 3550 m 3 /d is to be treated in a facultative pond system. The reaction rate coefficient at the average operating temperature is 0.35 d -1 . The pond system is assumed to behave...
-
Provide a brief overview using these environmental scanning steps as a guide: 1. Choose an industry of your interest such as technology, healthcare, retail, energy, etc. 2. Identify and briefly...
-
Your company is considering expanding into a new international market. Describe the market research you would conduct to evaluate the feasibility of entering this market, including factors such as...
-
Address the following from your Social Era research and the course scholarly literature: What are the key foundational underpinnings that shape the essence of what we call the social management era?...
-
Consider benzene (C6H6) in the gas phase. (a) Write the reaction for breaking all the bonds in C6H6 (g), and use data in Appendix C to determine the enthalpy change for this reaction. (b) Write a...
-
Refer to the Conservation Ecology (Dec. 2003) study of the causes of forest fragmentation, presented in Exercise 2.166 (p. 97). Recall that the researchers used advanced high-resolution satellite...
-
Devise a block-and-tackle arrangement that amplifies the applied force by a factor of three.
-
Imagine that you are a passenger on the International Space Station. While on the station, you are weightless and so is everything else, including wrenches and furniture. Even though everything...
-
Imagine a skydiver who waits a long time before opening her parachute. For simplicity, assume she moves along a straight line. (a) In what direction is the skydiver moving (what is the direction of...
-
DISCUSSION ACTIVITY All jurisdictions have legislation protecting seniority and benefits for qualified employees who are members of the Canadian Forces Reserves and who are deployed for active...
-
Firm J has net income of $90,160, sales of $980,000, and average total assets of $490,000. Firm J has net income of $90,160, sales of $980,000, and average total assets of $490,000. Required:...
-
Read Chapter 5 and the Tyco case and identify some of the signals of the misuse of acquisitions or merger reserves. How could these signals have helped the users of Tyco's financial statements...

Study smarter with the SolutionInn App


