Calculate the enthalpy of reaction for the reaction of N 2 and H 2 to form 2
Question:
Calculate the enthalpy of reaction for the reaction of N2 and H2 to form 2 mol NH3, using data from Table 9.4.
Table 9.4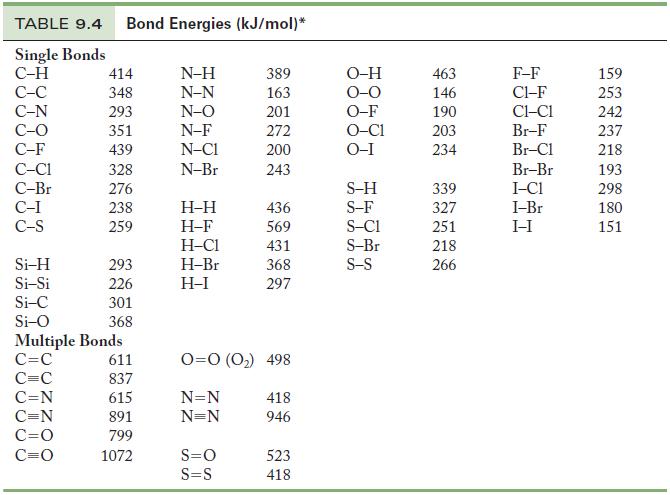
Transcribed Image Text:
TABLE 9.4 Bond Energies (kJ/mol)* Single Bonds C-H C-C C-N C-O C-F C-C1 C-Br C-I C-S Si-H Si-Si Si-C Si-O 414 348 293 351 439 328 276 238 259 293 226 301 368 Multiple Bonds C=C C=C C=N C=N C=O C=O 611 837 615 891 799 1072 N-H N-N N-O N-F N-CI N-Br H-H H-F H-C1 H-Br H-I 389 163 201 272 200 243 S=O S=S 436 569 431 368 297 0=0 (0₂) 498 N=N N=N 418 946 523 418 O-H O-F O-C1 O-I S-H S-F S-C1 S-Br S-S 463 146 190 203 234 339 327 251 218 266 F-F C1-F CHC1 Br-F Br-Cl Br-Br I-C1 I-Br I-I 159 253 242 237 218 193 298 180 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)

Answered By

Rajat Gupta
used to take tution classes from my school time.
Conducted special topic claases during my graduation to help the students pass their exams.
Currently, teaching and conducting online claases during my post- graduation too.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
In Exercise solve the given equations and check the results. 2 B4 1 B-2 1 2B + 4
-
Calculate the enthalpy of reaction for HCN(g) H(g) + C(g) + N(g) from enthalpies of formation (see Appendix C). Given that the CH bond energy is 411 kJ/mol, obtain a value for the C¡N bond...
-
For the reaction (a) Predict the enthalpy of reaction from the average bond enthalpies in Table 9.4. (b) Calculate the enthalpy of reaction from the standard enthalpies of formation (see Appendix 3)...
-
Jimmy and Elizabeth plan on getting married on the 15th of August. Elizabeth decides that she would prefer to have the reception in her backyard, so she rents an event tent from CELEBRATE Ltd, a firm...
-
An object on the end of a string moves in a circle. The force exerted by the string has units of ML/T2 and depends on the mass of the object, its speed, and the radius of the circle. What combination...
-
A circular current loop of radius R carrying a current I is centered at the origin with its axis along the x axis. Its current is such that it produces a magnetic field in the positive x direction....
-
In the simple consumption-based asset pricing model, the growth rate of aggregate consumption is assumed to have a constant expectation and standard deviation (volatility). For example, in the...
-
Network Solutions just introduced a new, fully automated manufacturing plant that produces 2,000 wireless routers per day with materials costs of $50 per router and no other costs. The average number...
-
The owner of Atlantic City Confectionary is considering the purchase of a new semiautomatic candy machine. The machine will cost $24,000 and last 10 years. The machine is expected to have no salvage...
-
Draw the important resonance structures for chloric acid, HClO 3 .
-
Describe what is meant by resonance structures.
-
Write each sentence in Problems 2946 as a proportion, and then solve to answer the question. What number is 15% of 64?
-
Coronado Corporation accumulates the following data relative to jobs started and finished during the month of June 2025. Costs and Production Data Actual Standard Raw materials unit cost $2.20 $2.10...
-
Consider the following labor statistics for the adult population (age 16 and older) in Norway displayed in the table below (all numbers in millions). Employed 110 13 Not in Labor Force 75 Unemployed...
-
Blossom Variety Store uses the LIFO retail inventory method. Information relating to the computation of the inventory at December 31, 2026, follows: Cost Retail Inventory, January 1, 2026 $147,000...
-
Use polynomial division to show that the general expression for the factors of the difference of two cubes, x 3 - y 3 = ( x - y ) ( x 2 + xy + y 2 ) , is correct
-
A beam has a lenght of L = 9 m long and carrying the uniformly distributed load of w = 3 kN/m. w kN/m A RA a) Calculate the reaction at A. RA= KN L RB b) Calculate the maximum bending moment. Mmax=...
-
As stated in Chapter 23, mammalian cells can become resistant to the lethal action of methotrexate by the selective survival of cells containing increases in dihydrofolate reductase gene copy number...
-
Consider the combustion of methanol below. If 64 grams of methanol reacts with 160 grams of oxygen, what is the CHANGE in volume at STP. 2CH3OH(g) + 3O2(g) 2CO2(g) + 4H2O(1) The volume decreases by...
-
A lecithin was hydrolyzed to yield two equivalents of myristic acid. (a) Draw the structure of the lecithin. (b) This compound is chiral, but only one enantiomer predominates in nature. Draw the...
-
A cephalin was hydrolyzed to yield one equivalent of palmitic acid and one equivalent of oleic acid. (a) Draw two possible structures of the cephalin. (b) If the phosphodiester was located at C2 of...
-
Draw the resonance structures of a fully deprotonated phosphatidic acid.
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


