Calculate the mass of ammonium chloride that must be added to 500.0 mL of 0.32 M NH
Question:
Calculate the mass of ammonium chloride that must be added to 500.0 mL of 0.32 M NH3 to prepare a pH 8.50 buffer; Kb for NH3 is 1.8 × 10-5.
Strategy We need to calculate the amount of the acid (NH4+ ions from NH4Cl) that we must add to prepare an ammonium/ammonia buffer of pH 8.50. It will be easiest if we use the Henderson–Hasselbalch equation in terms of the number of moles of acid and base.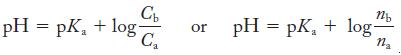
We know the pH, the value for Kb, and the volume and concentration of the base. To solve for na, we will also need to know Ka (and pKa), but we can calculate these values from Kb.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





