Calculate w for the following reactions that occur at 298 K and 1 atm pressure. Consider only
Question:
Calculate w for the following reactions that occur at 298 K and 1 atm pressure. Consider only PV work from the change in volume of gas, and assume that the gases are ideal and the chemical equation represents amounts in moles.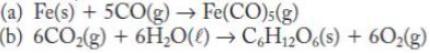
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a 98...View the full answer

Answered By

Collins Omondi
I have been an academic and content writer for at least 6 years, working on different academic fields including accounting, political science, technology, law, and nursing in addition to those earlier listed under my education background.
I have a Bachelor’s degree in Commerce (Accounting option), and vast knowledge in various academic fields Finance, Economics, Marketing, Management, Social Science, Women and Gender, Business law, and Statistics among others.
4.80+
4+ Reviews
16+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculate w for the following reactions that occur at 298 K and 1 atm pressure. Consider only PV work from the change in volume of gas, and assume that the gases are ideal and the chemical equation...
-
Calculate w for the following reactions that occur at 298 K and 1 atm pressure. Consider only PV work from the change in volume of gas, and assume that the gases are ideal and the chemical equation...
-
Calculate w for the following reactions that occur at 298 K and 1 atm pressure. Consider only PV work from the change in volume of gas, and assume that the gases are ideal and the chemical equation...
-
In an organization, managers communicate information downward to their departments and teams, and employees communicate information upward to their managers. If all members of an organization are not...
-
Predict the product(s) of the followingreactions: (b) 1. Nat "OEt Co (a) 2. CH3I eat (c) Br2, PBr3 H20 CH-CH2C (d) " NaOH, H20 12
-
Nicole Nelson has come into an inheritance from her grandparents. She is attempting to decide among several investment alternatives. The return after one year is dependent primarily on the interest...
-
Do we have procedures in place for getting employee input on ideas?
-
Aragon makes handheld calculators in two modelsbasic and professionaland wants to refine its costing system by allocating overhead using Professional, total OH $644,000 departmental rates. The...
-
A couple has decided to purchase a $140000 house using a down payment of $20000. They can amortize the balance at 9% over 20 years. a) What is their monthly payment? Answer = $ b) What is the total...
-
How much work is done if a balloon expands from 1.05 to 13.8 L against a constant external pressure of 1.08 atm?
-
What is the sign of w for the following processes if they occur at constant pressure? Consider only PV work from gases, and assume that all gases behave ideally.
-
The accountant for the Orion Sales Company is preparing the income statement for 2007 and the balance sheet at December 31, 2007. Orion uses the periodic inventory system. The January 1, 2007...
-
Joint Ventures are a common Mode of Entry in international business. Appreciate if in-depth elaboration provided on its advantages and disadvantages. Also briefly mention the factors which make joint...
-
The field excursion is intended to give students an opportunity to carry out an applied geographical research project based on observation, data recording, and analysis. Using a field site of your...
-
An angry coworker is expressing their needs through a rush of emotion and snide comments while another coworker is trying to interpret them to provide some help and support. You are a manager and...
-
You may have a general understanding of the difference between ethics and legality , but could you explain the distinction? It is not always easy to know where to draw the line between the two. Some...
-
Someone can be a good leader but not be a very good manager and vice-versa. Leadership is creating a vision for others to follow, establishing corporate values and ethics, and transforming the way...
-
Consider the two waves shown here, which we will consider to represent two electromagnetic radiations: (a) What is the wavelength of wave A? Of wave B? (b) What is the frequency of wave A? Of wave B?...
-
What can scientists learn by comparing the fossilized skeletons of extinct primates with the bones of modern species?
-
Determine v 1 and v 2 in the circuit of Fig. 3.101 . 20 10 10 100 V 10 10 20
-
If the Schematics Netlist for a network is as follows, draw the network. 1 2 2K 2 0 4K 3 0 8K R_R1 R_R2 R_R3 3 4 6K 1 3 4 0 DC 0 1 DC 1 3 VF F1 2 R_R4 R_R5 V_VS I_IS 100 4 F_F1 VF_F1 2 1 E_E1 3 3.
-
What is the basic distinction between the scientific method and other ways of looking at the natural world?
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


