Chlorine can be produced in the laboratory by the reaction of hydrochloric acid with excess manganese(IV) oxide.
Question:
Chlorine can be produced in the laboratory by the reaction of hydrochloric acid with excess manganese(IV) oxide.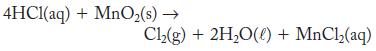
How many moles of HCl are needed to form 12.5 mol Cl2?
Transcribed Image Text:
4HCl(aq) + MnO₂(s) → Cl₂(g) + 2H₂O(0) + MnCl₂(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To find out how many moles of HCl are needed to form 125 moles of Cl2 we can use the stoi...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
L.W., a 20-year-old college student, comes to the university health clinic for a pregnancy test. She has been sexually active with her boyfriend of 6 months, and her menstrual period is now 2 weeks...
-
The reaction between potassium superoxide, KO2, and CO2, 4 KO2 + 2 CO2 2K2CO3 + 3 O2 is used as a source of O2 and absorber of CO2 in self-contained breathing equipment used by rescue workers. (a)...
-
Malic acid (C 4 H 6 O 5 ) is one of the alpha hydroxy acids which is naturally found in apples. The reaction of hydrochloric acid with sodium maleate to form malic acid and sodium chloride is...
-
What are the pros and cons of using social media at work? Discuss.
-
An ice skater of mass 75 kg is spinning about a vertical axis through the centre of her body, with arms outstretched horizontally, at a rate of two rotations per second. She then very quickly pulls...
-
Major financing and investing activities sometimes do not change cash. True or False?
-
Charisma of top-level leaders. Refer to the Academy of Management Journal (August 2015) study of the charisma of top leaders in business, Exercise 12.29 (p. 735). Recall that data were collected on...
-
Granite Engineering Ltd. has entered into a contract beginning January 1, 2013, to build a bridge in Tuktoyuktuk Shores. It estimates that the bridge will cost $14.8 million and will take three years...
-
Shadee Corporation expects to sell 6 3 0 sun shades in May and 3 3 0 in June. Each shade sells for $ 1 6 3 . Shadee's beginning and ending finished goods inventories for May are 6 5 and 5 5 shades,...
-
A. J. Smith Company started business on January 1, 2024, and the following transactions occurred in its first year: 1. On January 1, the company issued 12,000 common shares at $25 per share. 2. On...
-
Aluminum metal reacts with sulfuric acid, H 2 SO 4 , to yield aluminum sulfate and hydrogen gas. Calculate the mass of aluminum metal needed to produce 13.2 g hydrogen.
-
What mass of NH 3 forms from the reaction of 5.33 g N 2 with excess H 2 ?
-
Find three bases of R 2 .
-
Q. Probabilities of three teams A, B and C of winning the first prize of a business case competition are 3/9, 2/9, 3/9 respectively. These three teams are equally likely to win the prize. True False...
-
Univex is a calendar year, accrual basis retail business. Univex hold less than 20% of IBM stock. Its financial statements provide the following information for the year: Revenues from sales of goods...
-
Consider the unadjusted trial balance of Fabuloso, Inc. at December 31, 2023, and the related month-end adjustment data. (Click the icon to view the month-end adjustment data.) Requirements 1....
-
5.3 BEP Example Bill Braddock is considering opening a Fast 'n Clean Car Service Center. He estimates that the following costs will be incurred during his first year of operations: Rent $9,200,...
-
The following is the text for an opinion on internal control for W Company, an issuer. Some words or phrases have been replaced with numbers (e.g., [1], [2], etc.). Select from the option list...
-
The following unadjusted accounts are taken from the records of B Corp. at December 31, 2016: Additional Information: Interest expense for the year should be $1,200. Required: Prepare the adjusting...
-
Explain the term global capital markets. This chapter primarily discusses global equity markets. What other types of financial instruments are traded in these markets? How important are global...
-
Will an S N 1 process involving each of the following nucleophiles require a proton transfer at the end of the mechanism? (a) NaSH (b) H 2 S (c) H 2 O (d) EtOH (e) NaCN (f) NaCl (g) NaNH 2 (h) NH 3...
-
For each of the following substrates, determine whether an S N 1 process will involve a carbocation rearrangement or not: (a) (b) (c) (d) (e) (f)
-
Draw the skill the mechanism for each of the following S N 1 processes: (a) (b) (c) (d) (e) (f) (g) (h) Br HBr HBr -Br
-
During 2024, its first year of operations, Hollis Industries recorded sales of $11,900,000 and experienced returns of $760,000. Cost of goods sold totaled $7,140,000 (60% of sales). The company...
-
What is the value of a 15% coupon bond with 11% return? Is it a discount or a premium bond?
-
A manufacturer with a December 31 taxation year end sells new machinery for $50,000 on January 2, 2022. The cost of the machinery is $20,000. The terms of the sale require an initial payment of...

Study smarter with the SolutionInn App


