Determine the entropy change when 1 mol H 2 O boils at its normal boiling point of
Question:
Determine the entropy change when 1 mol H2O boils at its normal boiling point of 100.0 °C . Th e heat of vaporization of water is 44.0 kJ/mol .
Strategy
We will use Equation 17.5, remembering that when the temperature variable is used in this equation, it must be in kelvin units.
Equation 17.5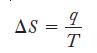
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Because we have 1 mol H 2 O the heat i...View the full answer

Answered By

AJIN kuriakose
I have completed B.Tech in Electrical Engineering & Masters in Power & Control From one of the best universities in India. I got the 99.05 percentile in the Gate Electrical Engineering Exam. I can Help students solving assignments in Electrical subjects like Power Electronics, Control system, Analog, Network Theory & Engineering Mathematics. Clear your fundamentals and develop problem-solving skills and analytical skills to crack the exam.
Get guidance and the opportunity to learn from experienced...
I can provide tuition for Electrical engineering subjects (Power Electronics, Digital electronics, Network Theory, Control System & Engineering Mathematics). The toughest subject of Electrical engineering can be made simple in online classes...
I can also solve it.
1 .I can help you with your assignments or exams or quiz or tutoring.
2. Very strict to the deadlines.
Message me for any help in assignments, live sessions. I am here to help students for all assignments, tests and exams and I will make sure you always get _95% In your subject.
Contact me in solution inn for any help in your semester, projects and for many more things . Also feel free to contact me through solution inn and for any advise related to tutoring and how it works here.thank you.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The enthalpy of vaporization of methanol is 35.27 k] mol-I at its normal boiling point of 64.1oC. Calculate (a) The entropy of vaporization of methanol at this temperature and (b) The entropy change...
-
Determine the entropy change when 1 mol H 2 O freezes at its normal freezing point of 0.0 C. Th e heat of fusion of water is 6.01 kJ/mol.
-
The normal boiling point of Br2(l) is 58.8C, and its molar enthalpy of vaporization is Hvap = 29.6 kJ/mol. (a) When Br2(l) boils at its normal boiling point, does its entropy increase or decrease?...
-
Explain factors governing recruitment?
-
Tert-Butoxycarbonyl azide, a reagent used in protein synthesis, is prepared by treating tert-Butoxycarbonyl chloride with sodium azide. Propose a mechanism for thisreaction. , H3C CH3 + NaN3 NacI ...
-
Select an everyday project you are familiar with such as a class project, preparing a meal, making a pizza, repairing your car. Develop a list of the activities, a CPM/PERT network (with time...
-
How is product quality, and how does it compare with that of competitors?
-
Complex wave vectors in the energy gap find an expression for the imaginary part of the wave vector in the energy gap at the boundary of the first Brillouin zone, in the approximation that led to Eq....
-
how can I get plus: desired materials in ending inventory? same for less direct materials in beginning inventory Points: 0 of 1 Preston, Inc. manufactures model airplane kits and projects production...
-
Calculate the standard entropy change when 1 mol of propane burns in oxygen. Strategy Use the standard molar entropies from Appendix G. For each product and reactant, do not forget to multiply the...
-
E is -2.21 10 3 kJ/mol for the combustion of propane. Calculate H for this reaction at 25 C and 1.00 atm . Strategy Use Equations 17.3 and 17.4 to determine H. The change in the number of moles of...
-
Discuses the challenges of getting managers to think strategically.
-
Systems thinking is all about solving problemsin organizations, world situations, and even our personal lives. But it is not just a procedure; it is a different way of approaching problems. Our...
-
How would I display the following 3 principles in an entertaining infographic? Be very specific . Principle 1: Employee Engagement and Motivation Drawing from the Human Relations Movement theory and...
-
Shown below is a cross section of tubular member which is subjected to a torque T= 5.5 kN-m. It has a length L-3.0-m and the material shear modulus G=27 GPa. Dimensions: b=150 mm, h= 100 mm and t= 8...
-
The hip roof shown in the below Figure 2 is constructed of 2x10 rafters spaced 16 inches on center. The hip rafters are 1 -inch-wide by 12-inch-high GLBs. The roof has a slope of 4:12. Prepare a list...
-
2. Estimate the populations of Fargo, ND and Bismarck, ND in years of 2040 and 2050. Select a single value of population that you would use for design purposes in each year. You need to specify and...
-
One type of sunburn occurs on exposure to UV light of wavelength in the vicinity of 325 nm. (a) What is the energy of a photon of this wavelength? (b) What is the energy of a mole of these photons?...
-
In Problem use absolute value on a graphing calculator to find the area between the curve and the x axis over the given interval. Find answers to two decimal places. y = x 3 ln x; 0.1 x 3.1
-
For the flow of water in the square tubes described in Problem 9.32, compute the pressure drop over a length of 22.6 m. All surfaces are copper and the duct is horizontal.
-
For the glycerin described in Problem 9.31, compute the pressure drop for a horizontal duct 22.6 m long. All surfaces are copper.
-
For the heat exchanger described in Problem 9.29, compute the pressure drop for a length of 57 in.
-
Ellis Perry is an electronics components manufacturer. Information about the company's two products follows: \ table [ [ , , , ] , [ Units produced,AM - 2 , FM - 9 , ] , [ Direct labor hours required...
-
Which of the following requirements to claim Earned Income Tax Credit is TRUE? The credit can be claimed under any filing status. The taxpayer must have a valid SSN for employment in the U.S., issued...
-
Olde Tyme Beverage Companys operating activities for the year are listed below. Cost of Goods Manufactured $131,000 Operating expenses 80,000 Beginning inventory, FG 16,000 Ending inventory, FG...
The Crypto Code Unlocking The Power Of Virtual Assets 1st Edition - ISBN: 979-8397863490 - Free Book

Study smarter with the SolutionInn App


