Determine whether each of the following reactions favors the reactants, products, or neither.
Question:
Determine whether each of the following reactions favors the reactants, products, or neither.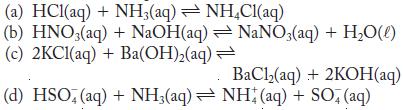
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The provided image shows a list of chemical reactions and youre asking about which direction each of the reactions favors toward the reactants product...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Tell whether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants and products are soluble.) (a) CHCl + F- (b) CH3CI + N3 (c) CHCl + OCH3 CHF+CI-...
-
Tell wbether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants and products are soluble.) (a) CH3CI + I- CH3I + CI- (b) CH3CI + -OCH3 CH3OCH3 +...
-
Determine whether each of the following reactions favors the reactants, products, or neither.
-
2. The beam in the figure is made of material having modulus of elasticity E = 200 GPa . The moment of inertia of segments AB and CD of the beam is I = 6.77 10-6 m, while the moment of inertia of...
-
? - Farnesene is a constituent of the natural wax found on apples. What is its IUPAC name, including stereo chemistry? a-Farnesene
-
Distinguish between the following payroll audit procedures and state the purpose of each: (1) Trace a random sample of prenumbered time cards to the related payments in the payroll register and...
-
Account for equity securities with significant influence. (p. C-7) AppendixLO1
-
Weller Company's flexible budget for manufacturing overhead (in condensed form) follows The following information is available for a recent period: a. The denominator activity of 8,000 machine-hours...
-
The argument that interstate banking would allow banks to grow and more fully achieve a reduction in operating costs per unit of output as output increases is based on: a. economies of scale. b....
-
A solution is made by diluting 10.0 mL of concentrated ammonia (28% by weight; density = 0.90 g/mL) to exactly 1 L. Calculate the pH of the solution.
-
Calculate the pH of 0.25 M solutions of the following solutes. (a) Hydrofluoric acid (b) Potassium fluoride
-
Show that for an integer , (1.2.2) immediately follows from (1.2.1). (Advice: Think about N as the moment of the th success.)
-
Directions: Put your feet in the shoes of the business owner and suggest specific ways on how a business can gain profit and how it can be avoid loss Ways to Gain Profit 1. 2. 3. 4. 5. 1. 2. 3. 4. 5....
-
Define business intelligence Briefly discuss how your organisation can use business intelligence to improve decision-making Please see below rubric as guidance. Kindly list references in APA 7th...
-
Mr Santos apply for college educational plan for his 3 children .The 3 children ages are 6 yrs old , 3 yrs old and 1 yr old. The fund will be set-up the deposit of a fixed sum on the child's current...
-
Recovery Centers of America needs to acquire new vehicles that will cost $2.5 million across its six state service area. It plans to use the vehicles for three years, at which time new vehicles will...
-
The following program is supposed to allow the user to enter a list of numbers, then print them out formatted with 2 decimal positions. However, there are 3 errors. Indicate the line number of each...
-
A 35.0-mL sample of 1.00 M KBr and a 60.0-mL sample of 0.600 M KBr are mixed. The solution is then heated to evaporate water until the total volume is 50.0 mL. What is the molarity of the KBr in the...
-
Place a tick in the appropriate grid to identify the balance that would be brought down in each of the following named accounts, in the books of Rizwy Mohamed: (a) In the Cash account: if Rizwy...
-
A popular circus act features daredevil motorcycle riders encased in the ???Globe of Death??? (Fig. P5.65), a spherical metal cage of diameter 16 ft.? (a) A rider of mass 65 kg on a 125-cc (95-kg)...
-
An ancient and deadly weapon, a sling consists of two braided cords, each about half an arm???s length long, attached to a leather pocket. The pocket is loaded with a projectile made of lead, carved...
-
A man stands 6.0 ft tall at sea level on the North Pole as shown in Figure P5.62.? (a) What is the difference in the value of g (the gravitational acceleration) between his head and his feet?? (b)...
-
4) Read the following case carefully and answer the given questions. You have been the finance director of a clothing retailer for ten years. The companys year end is 31st December 2019, and you are...
-
all of the other problems here on chegg don't describe right on how they god the answer. can you make it step by step math to show how you got what and from where and each number to get the answer...
-
D Required information The following Information applies to the questions displayed below.) Diego Company manufactures one product that is sold for $76 per unit in two geographic regions-the East and...

Study smarter with the SolutionInn App


